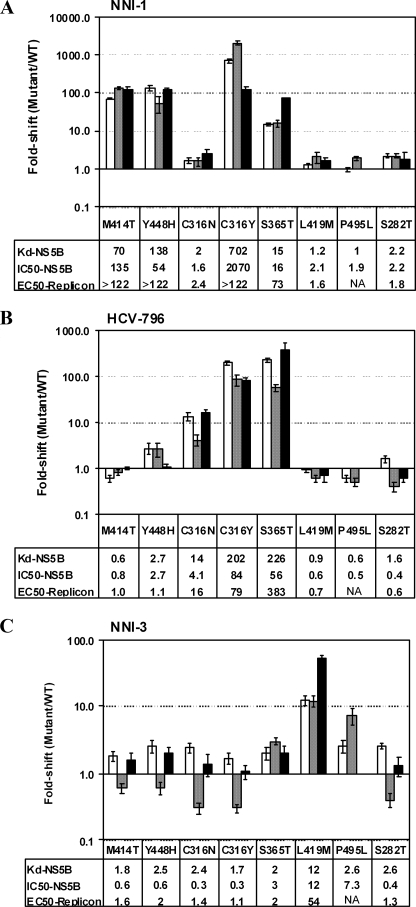
FIGURE 6.
Effects of palm and thumb resistance mutations on binding affinity of inhibitors to NS5B and the potency of the inhibitors to suppress polymerase activity and HCV replicon replication. A, NNI-1; B, HCV-796; C, NNI-3. Compound binding affinity and polymerase-inhibitory activity were tested on NS5B570-Con1 proteins carrying single mutations (M414T, Y448H, C316N, C316Y, S365T, L419M, P495L, or S282T) in the fluorescence quenching binding assay and HCV RNA polymerase assay. Inhibition on the HCV replicon assay was tested as described under “Experimental Procedures” in an HCV bicistronic system carrying the single mutations indicated above. -Fold changes in Kd of mutant proteins were calculated relative to the Kd value measured for wild-type NS5B570-Con1. -Fold changes in IC50 for NS5B and EC50 for HCV replication were calculated based on the respective IC50 values measured for wild-type NS5B protein or the EC50 values for HCV replication. EC50 values of HCV replication with a polymerase bearing the P495 mutation could not be measured because the compounds were toxic to the replicon. NA, not available. White bars, -fold changes in Kd; gray bars, -fold changes in IC50 for NS5B polymerase activity; black bars, -fold changes in EC50 for HCV replication.