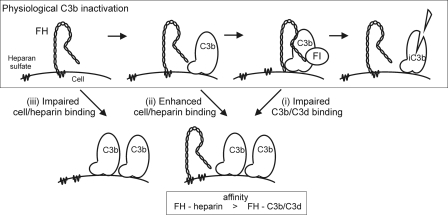
FIGURE 7.
Proposed mechanism of FH function on surface-bound C3b and the pathogenic mechanisms of aHUS associated with FH mutations. Under the physiological conditions FH may be attached to the cell surface GAGs such as heparan sulfate. When C3b is deposited onto the surface the C terminus of FH is released from the cell surface GAGs to bind to the C3d part of C3b. Thereafter the N terminus of FH acts efficiently as a cofactor for factor I (FI) in cleavage of C3b to inactive C3b (iC3b) (upper panel). The three primary binding defects of mutated FH in aHUS can lead to restricted C3b elimination via three basic mechanisms. (i) FH mutations that decrease C3b/C3d binding lead to impaired C3b elimination on a cell surface (right lower panel). (ii) If FH binding to GAGs on a cell surface is clearly increased, the ability of the mutated FH molecules to bind to C3b is decreased due to the overlapping binding sites. Thereby inactivation of C3b can be ineffective (right lower panel). (iii) If affinity of FH to the cell surface GAGs is impaired the deposited C3b may not be efficiently inactivated due to lack of FH in close proximity (left lower panel). Both mechanisms i and ii are associated with a relative decrease of FH affinity for C3b/C3d compared with heparin.