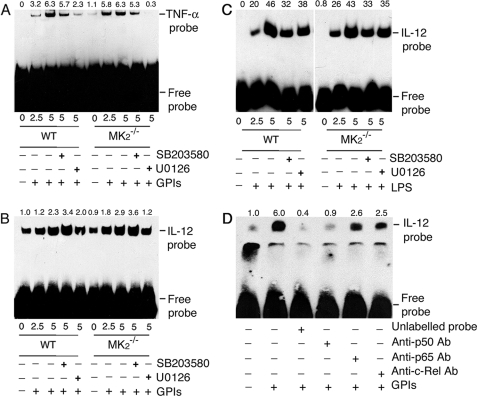
FIGURE 8.
EMSA analysis of NF-κB binding to TNF-α and IL-12p40 gene promoter in macrophages stimulated with GPIs and LPS. WT and MK2−/− macrophages (∼2 × 106 cells/flask) were stimulated with 200 nm GPIs for the indicated time periods. Both types of macrophages, pretreated with either 10 μm U0126 or 10 μm SB203580 for 1 h, were also stimulated with either 200 nm GPIs or 100 μg/ml LPS. Unstimulated cells were used as controls. The cells were harvested and nuclear extracts prepared (50 μl total volume), and 2 μl of each extract was incubated with biotin-labeled TNF-α κB DNA probe (A) or IL-12p40 κB DNA probe (B, GPI-stimulated cells; C, LPS-stimulated cells) in binding buffer (20 μl) as described under “Experimental Procedure.” The reaction mixtures were electrophoresed on 6% polyacrylamide gels, and protein bands were transferred onto nylon membrane and detected with chemiluminescent nucleic acid detection reagent. D, NF-κB proteins that bound to IL-12p40 κB DNA probe were identified by inhibition with specific antibodies. The nuclear extracts from GPI-stimulated macrophages were incubated with 2 μg each of anti-p50, anti-p65, or anti-c-Rel antibody or 5 pmol (100-fold excess over labeled probe) of unlabeled κB probe prior to the addition of biotin-labeled IL-12p40 κB probe. The samples were analyzed as above. The relative band intensities of specific NF-κB-DNA complex, determined by scanning of x-ray films using Bio-Rad GS-800 densitometer, are shown on the top of each electrophoretogram.