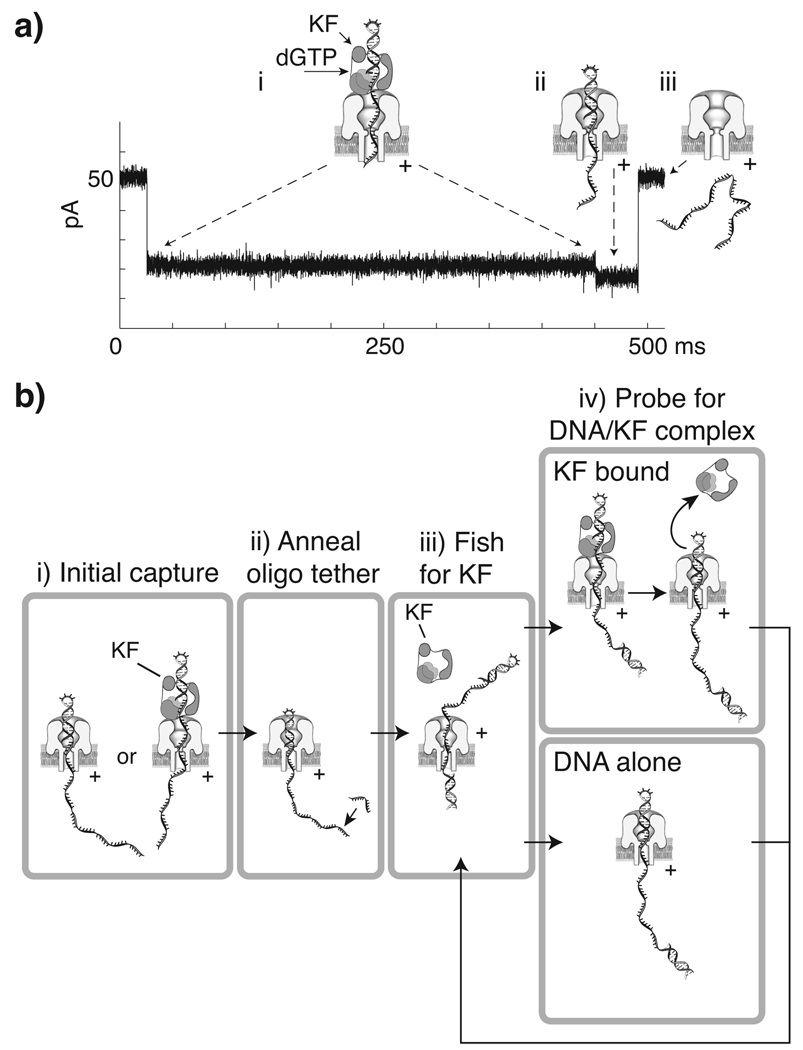
Figure 2. Use of the two step current signature of DNA-KF and DNA-KF-dNTP complexes to control KF association and dissociation from a DNA substrate tethered in the nanopore.
a) Representative current trace and illustration of corresponding molecular events during nanopore capture of a DNA-KF-dGTP ternary complex. The initial ~21.5 pA blockade (i) arises from capture of the enzyme bound DNA, with the duplex held atop the pore vestibule bound to KF. The shorter ~18.5 pA terminal step (ii) occurs upon KF dissociation, when duplex DNA is pulled into the nanopore vestibule, followed by (iii) duplex unzipping and translocation of the DNA through the nanopore, and return to the open channel current. b) Strategy for control of iterative KF association and dissociation from a nanopore-tethered DNA molecule. (i) A DNA hairpin, either KF bound or unbound, is captured under applied voltage. When the amplitude level that distinguishes capture of DNA alone or the terminal step of KF bound events is detected, (ii) the FSM lowers the voltage to hold the hairpin duplex in the pore vestibule long enough to anneal an oligonucleotide to the single-stranded end protruding into the trans chamber. With the DNA tethered in the pore by duplexes at both ends, a negative voltage is applied (iii) to expose the DNA to KF in the cis chamber (‘fishing’). After a programmed time period, the voltage is reversed (iv) to draw the duplex back to the pore and diagnose whether it is KF bound based upon amplitude (‘probing’). Detection of unbound DNA prompts a return to the fishing voltage. Diagnosis of the KF bound state results in continued application of the probing voltage until the terminal step is detected, which then prompts a return to fishing.