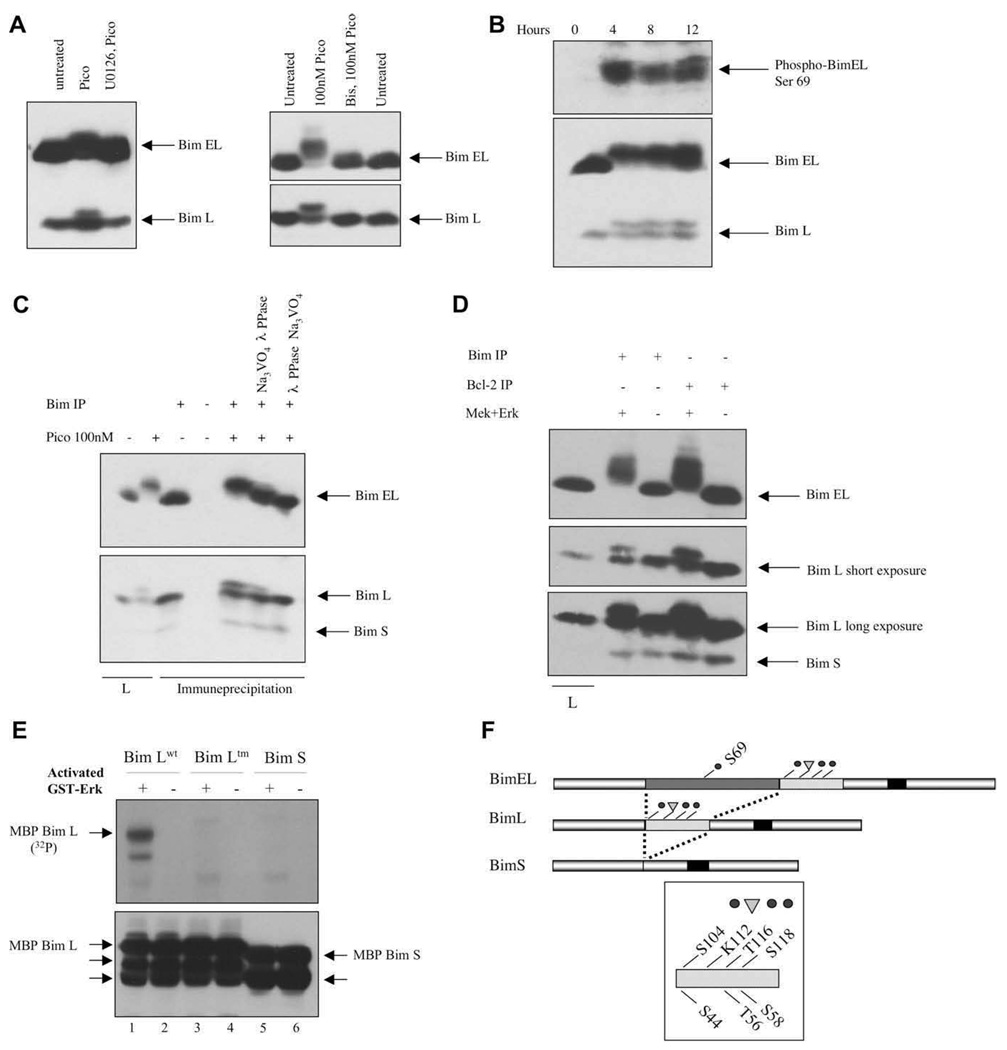
Figure 4.
Phosphorylation of BimEL and BimL by Erk in vivo and in vitro. (A) Cells were treated with 10 nM pico for 28 hours (left panel) or 100 nM for 20 minutes (right panel). Inhibitors were added prior to diacylglycerol (DAG) analogue stimulation. (B) Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells were treated for various periods of time with 10 nM pico and Bim phosphorylation was demonstrated either by probing with an anti-phospho-BimEL (Ser 69) antibody (top panel) or by electrophoretic mobility shifting (bottom panel). (C) The reduced electrophoretic mobility of BimEL and BimL was reversed by in vitro phosphatase treatment. The first two lanes represent lysates (L) analyzed directly. (D) Endogenous Bim proteins, immune-precipitated directly or co-precipitated with anti–Bcl-2 antibodies, were phosphorylated with recombinant Mek plus Erk before electrophoretic mobility shift analysis. (E) Recombinant maltose-binding protein (MBP)-BimL wild-type (wt), MBP-BimL triple mutant (tm), and MBP-BimS were incubated with Mek-activated Erk (odd numbered lanes) in the presence of labeled adenosine triphosphate (ATP). Top panel, radioactive phosphate incorporation was detected by 2 hours autoradiography. Bottom panel, MBP-BimL and MBP-BimS were detected by immunoblotting with an anti-Bim antibody. Unlabeled arrows indicate proteolytic breakdown products of MBP-Bim that preexisted in the protein preparations prior to the kinase assay. Control lanes (even numbers) were from identical paired reactions, but Erk was omitted. (F) Schematic diagram showing the structural relationships between the major Bim splice forms and the positions of the phosphorylation sites and the potential ubiquitination site (K112) discussed in the text.