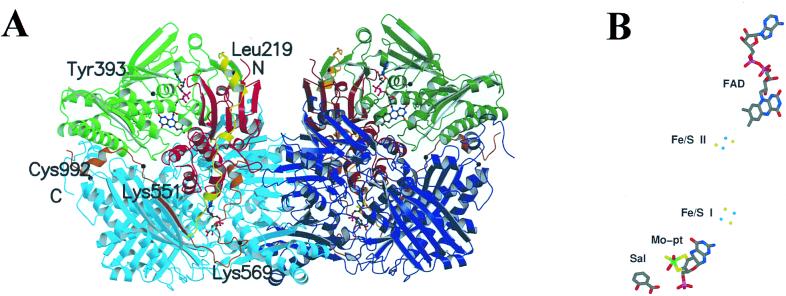
Figure 1.
(A) Molecular structure of the XDH dimer divided into the three major domains and two connecting loops. The two monomers have symmetry related domains in the same colors, in lighter shades for the monomer on the left and in darker shades for the monomer on the right. From N to C terminus, the domains are: iron/sulfur-center domain (residues 3–165; red), FAD domain (residues 226–531; green), and Mo-pt domain (residues 590–1,331; blue). The loop connecting the iron/sulfur domain with the FAD domain (residues 192–225) is shown in yellow, the one connecting the FAD domain with the Mo-pt domain (residues 537–589) is in brown, and the N and C termini are labeled. The FAD cofactor, the two iron/sulfur centers, the molybdopterin cofactor, and the salicylate also are included. The positions of residues discussed in the text are indicated. (B) For clarity, the arrangement of the cofactors and salicylate in one subunit of XDH are presented. The Mo ion is in green, the iron ions are in light blue, and the sulfur atoms in yellow.