Figure 1. Ex vivo dissection strategy.
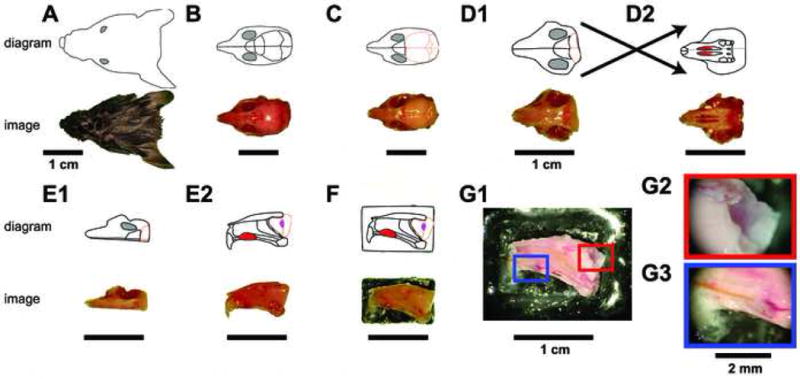
The connected VNO and AOB of adult male B6D2F1 mice were isolated by performing the diagrammed steps in ice-cold aCSF. For each step, a digital image is shown at the bottom, and a schematic diagram shown on top. Following decapitation (A), the lower jaw, scalp, and external sensory structures are removed from the skull (B). The skull overlying the cerebral cortex and cerebellum is then removed (C), followed by removal of the majority of the brain posterior to the olfactory bulbs (D1). The soft palate is then detached, revealing the two vomeronasal organs (D2 top, red oval structures). The bone surrounding the VNO capsule is broken on the contralateral hemisphere (not shown) using a scalpel point, followed by a careful hemispherectomy of the contralateral portion of the skull (E1). Upon turning the hemisphere on its lateral edge, the VNO can be seen from its medial face (E2 top, red oval), along with the septal cartilage and bone. The olfactory bulb (E2 top, peach outline) containing the accessory olfactory bulb (E2 top, magenta oval) can be visualized. The lateral face of the hemisphere is then affixed to a plastic plank via tissue adhesive (F) and placed into the perfusion chamber. (G) The final dissection steps are performed in the perfusion chamber at room temperature. Following removal of all contralateral tissue, attached ipsilateral frontal cortex, and the septal bone and cartilage (G1), the AOB becomes visible (G2) and a stimulus cannula is threaded under visual control into the VNO lumen (G3).
