Figure 2. Ex vivo perfusion chamber schematic.
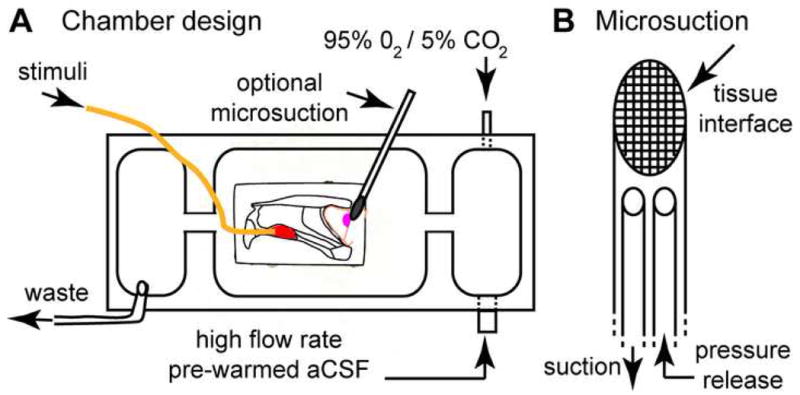
(A) VNO-AOB ex vivo preparations were placed in a custom tissue perfusion chamber designed per this schematic. Warmed, oxygenated aCSF is driven at ∼7.5 mL/min via a peristaltic perfusion pump (not shown) into a small antechamber (right). In this antechamber, the perfusion aCSF was re-oxygenated with 95% O2/5% CO2. This oxygen-rich aCSF entered the main chamber via an opening near the AOB (magenta oval). In some experiments, a small microsuction device was placed at the lateral face (i.e. the surface facing downward into the page) of the main olfactory bulb to encourage deep tissue perfusion. Stimuli were delivered into the VNO (red oval) through a polyimide cannula (orange line) at 0.2-0.4 mL/min. Waste solution was removed from a third chamber physically separated from the main chamber to reduce surface vibrations. (B) Detailed diagram of micro-suction device design. A series of fine nylon mesh screens (10-50 μm in diameter, with smallest pore size at tissue interface) were affixed to a polyimide suction wand. A pressure release cannula was threaded into the lumen of the device and left open to the superfusion solution in order to normalize suction pressure at the tissue interface over time.
