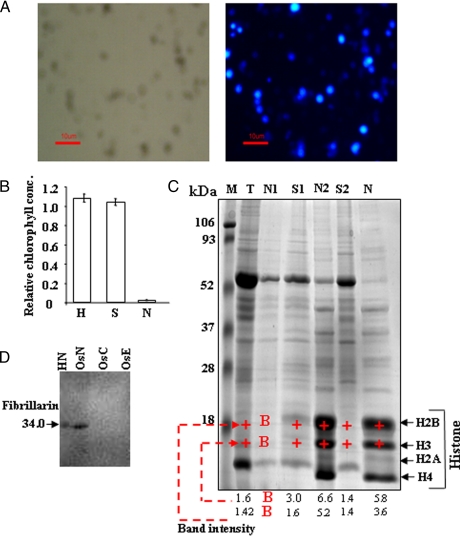
Fig. 3.
Analysis of rice nuclear fraction. A, the purified nuclear fraction was stained with DAPI and visualized by fluorescence microscopy. The phase-contrast micrograph of the nuclei is shown in the left panel; the DAPI-stained nuclei are shown in the right panel. B, determination of chlorophyll content at different stages of purification of nuclear fraction. The amounts of chlorophyll present in whole cell extracts (H), supernatant (S), and nuclear (N) fraction was estimated and compared. Data represent means ± S.D. of three replicates. C, representative analytical 1-D electrophoresis profile (12.5% SDS-PAGE, Coomassie Blue) obtained from rice protein extract prepared using HB. M, molecular mass protein standards; T, total cell extract; N1, nuclear protein without wash; S1, supernatant protein; N2 and S2, nuclear protein and supernatant protein, respectively, after first wash; N, final nuclear protein after another wash. The four abundant bands at around 10–18 kDa analyzed by MS were shown to contain histones H2B, H3, H2A, and H4. The intensity of the bands corresponding to histones H2B, H3 (+) was quantified by using the densitometry Quantity One 1-D software (Bio-Rad); B indicates a signal close to background levels. D, immunoblot analysis of extracted nuclear proteins with anti-fibrillarin antibodies. Aliquots of 100 μg of protein, each from nuclear (OsN), chloroplast (OsC), and extracellular matrix (OsE) fractions of rice as well as HeLa nuclear extract (HN), were separated by 12.5% SDS-PAGE. HeLa nuclear extract was used as positive control, whereas chloroplast and extracellular matrix fractions were used as negative controls. The 1-D gel was electroblotted onto Hybond-C membrane, and fibrillarin was detected using alkaline phosphatase-conjugated secondary antibody.