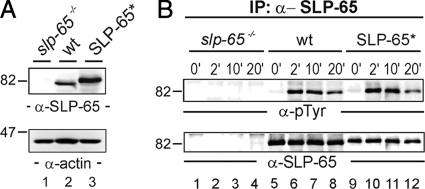
Fig. 1.
Functional reconstitution of BCR signaling by peptide-tagged SLP-65. A, cleared cellular lysates of SLP-65-deficient DT40 B cell mutants (slp-65−/−; lane 1), wild-type DT40 cells (wt; lane 2), and reconstituted DT40 mutants expressing a SLP-65 version harboring a One-STrEP peptide tag at the N-terminal end (SLP-65*; lane 3) were subjected to immunoblot analysis with antibodies against chicken SLP-65 (upper panel). Equal protein loading was ensured by anti-actin immunoblotting (lower panel). B, cells described in A were left untreated (0 min; lanes 1, 5, and 9) or stimulated through their BCR for 2 (lanes 2, 6, and 10), 10 (lanes 3, 7, and 11), and 20 min (lanes 4, 8, and 12). From the cleared cellular lysates, SLP-65 proteins were purified by anti-SLP-65 immunoprecipitation and analyzed by anti-phosphotyrosine or anti-chicken SLP-65 immunoblotting (upper and lower panel, respectively).