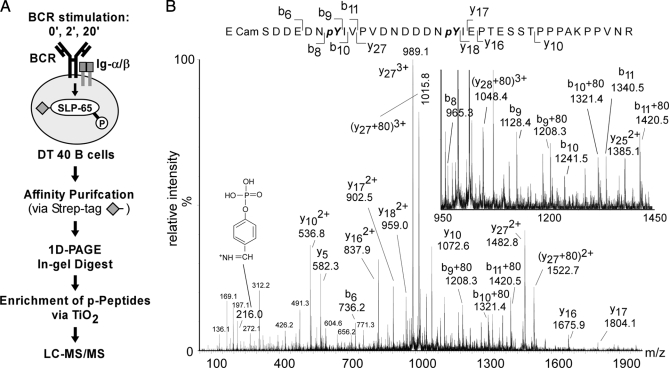
Fig. 2.
Identification of SLP-65 phospho-acceptor sites. A, strategy for proteomic analysis of in vivo phosphorylation sites in SLP-65. Following BCR stimulation of DT40 B cells, peptide-tagged SLP-65 was affinity-purified, subjected to 1D-PAGE, and digested in the isolated gel slice with trypsin or chymotrypsin. Phosphopeptide products were enriched by TiO2 chromatography and analyzed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). B, MS/MS spectrum of a quadruply charged and singly phosphorylated peptide (m/z 1461,9528) of SLP-65 in which tyrosines 194 (Y194) and 205 (Y205) are found to be phosphorylated (188SDDEDNpYIVPVDNDDDNpYIEPT ESSTPPPAKPPVNR223). Y-type and b-type fragment ions that identify unambiguously the two phosphorylation sites and the phosphotyrosine immonium ion (m/z = 216) are indicated.