Fig. 6.
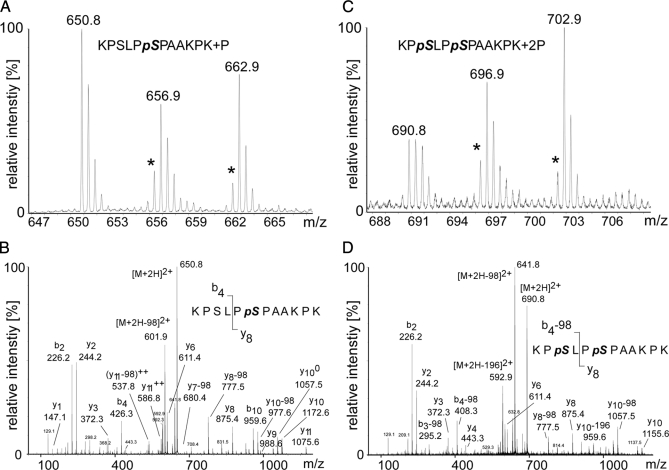
MS-based relative quantification of the most frequently detected SLP-65 phosphopeptide encompassing amino acids 168–179. A, MS spectrum of the singly phosphorylated peptide 168KPSLPpSPAAKPK179 (m/z = 650.8) containing Ser(P)-173 in unstimulated cells. Note the reduced peak intensity values for the peptides (m/z = 656.9, m/z = 662.9) obtained from cells stimulated through their BCR for 2 or 20 min, respectively. This indicates either BCR-induced dephosphorylation or conversion of the singly phosphorylated form into the doubly phosphorylated form. Intensities of mass peaks labeled with an asterisk might be slightly reduced due to a certain impurity of the isotope-labeled amino acids. B, Y-type and b-type fragment ions in the MS/MS spectrum show unambiguously that singly phosphorylated versions of the peptide 168–179 contain only Ser(P)-173 but not Ser(P)-170. C, MS spectrum of the doubly phosphorylated peptide (168KPpSLPpSPAAKPK179; m/z = 690.8) harboring both Ser(P)-170 and Ser(P)-173. Comparison of the peak intensities of unlabeled and labeled peptides reveals a stimulation-dependent enrichment of the doubly phosphorylated peptide indicating BCR-induced phosphorylation. D, Y-type and b-type fragment ions in the MS/MS spectrum of the doubly phosphorylated peptide 168KPpSLPpSPAAKPK179 unambiguously reveal that both serines 170 and 173 are phosphorylated.