Fig. 9.
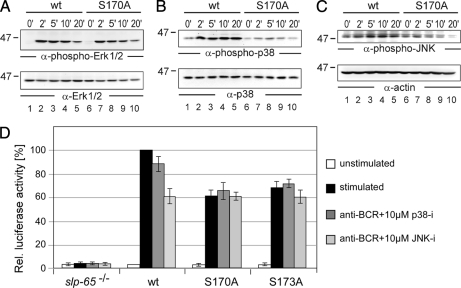
Phosphorylated serines 170 and 173 serve as a distal switch for the activation of MAP kinases p38/JNK and the AP1 transcription factor. DT40 B cells expressing GFP-tagged versions of wild-type SLP-65 (lanes 1–5) or S170A mutant SLP-65 (lanes 6–10) were investigated for their ability to activate distinct MAP kinase family members upon BCR engagement for the indicated times by immunoblot analysis of cleared cellular lysates with antibodies against (A) phospho-Erk, (B) phospho-p38, and (C) phospho-JNK (upper panels). Protein loading was monitored by immunoblotting with antibodies against Erk, p38, and actin, respectively (lower panels). D, SLP-65-deficient DT40 cells and those cells expressing wild-type SLP-65 or its mutant forms S170A or S173A were monitored for their ability to activate transcription of an AP1-driven luciferase reporter gene by co-transfection of an AP1 reporter construct with a β-galactosidase expression plasmid followed by determination of enzyme activities in untreated cells (white bars) and cells stimulated through their BCR for 6 h (black bars). Where indicated (dark gray and light gray bars), cells were pretreated with inhibitors of either p38 or JNK one hour before stimulation. Three independent experiments were performed, and data were normalized according to transfection efficiencies.