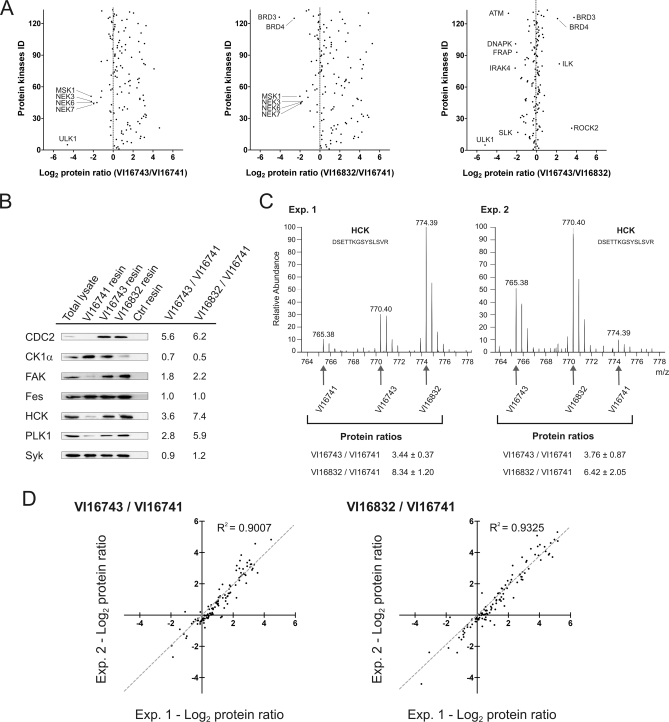
Fig. 2.
Quantitative analysis of protein kinases enriched with three different pyrido[2,3-d]pyrimidine ligands. A, relative protein kinase binding to the affinity resins was determined by SILAC-based quantitative MS of unique peptides. For the three pair-wise resin comparisons, the averaged ratios of two independent experiments were plotted on a logarithmic scale (log2) for all protein kinases. B, aliquots of MV4–11 cell lysate and eluted protein from incubations with the indicated inhibitor resins or control (Ctrl) beads were separated by SDS-PAGE and immunoblotted with antibodies recognizing the kinases CDC2, CK1α, FAK, Fes, HCK, PLK1, and Syk. For comparison, ratios of relative kinase binding according to the quantitative MS analysis are shown. C, MS spectra of a HCK peptide detected as a triplet due to SILAC encoding. Upon swapped SILAC labeling, relative ion intensities changed accordingly in replicate experiments. Peptide species derived from the different affinity purifications are marked by arrows, and the resulting ratios are shown for both experiments. D, log2 transformed ratios of kinase binding to the different resins are shown in scatter blot comparisons of two independent experiments. Pearson correlation coefficients (R2) close to one indicated an overall high concordance of measured kinase ratios in biological replicates.