Figure 6.
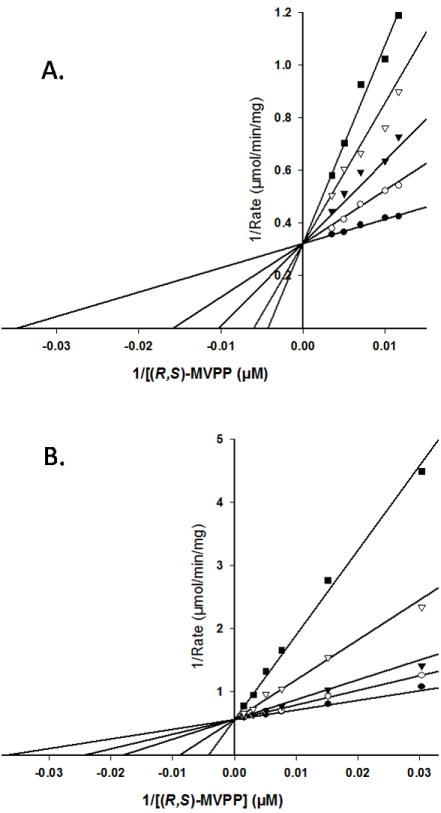
Competition for the mevalonate diphosphate site of wild-type human MDD. Double-reciprocal plots of the reaction velocity as a function of mevalonate 5-diphosphate concentration, measured at different levels of the inhibitors diphosphoglycolyl proline (A) or 6-fluoromevalonate 5-diphosphate (B). Data were fit to a competitive inhibition model using SigmaPlot 10.0/Enzyme Kinetics 1.3. (Systat Software, Inc.). Panel (A) displays the inhibition by diphosphoglycolyl proline measured at the following concentrations: (●) 0.0 mM, (○) 2.7μM, (▼) 5.5 μM, (▽) 11.0 μM, (■) 16.5 μM. Panel (B) displays the inhibition by 6-fluoromevalonate 5-diphosphate measured at the following concentrations: (●) 0.0 nM, (○) 32 nM, (▼) 65 nM, (▽) 194 nM, (■) 484 nM.
