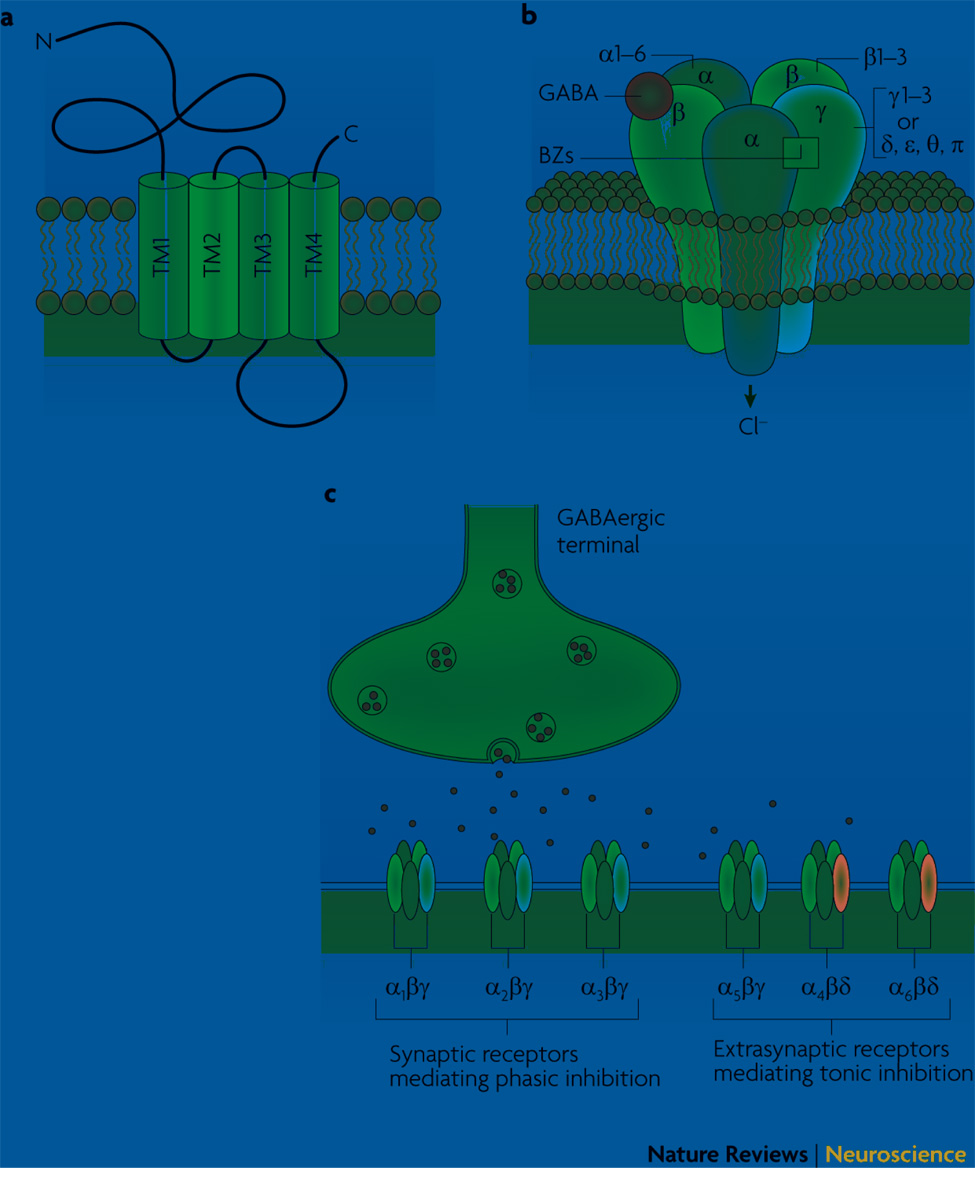
Figure 1. GABAA receptor structure and neuronal localization.
(A) GABAA receptors are members of the ligand-gated ion channel superfamily. Receptor subunits consist of four hydrophobic transmembrane (TM1–4) domains, where TM2 is believed to line the pore of the channel. The large extracellular N-terminus is the site for the binding of the neurotransmitter GABA, as well as containing binding sites for psychoactive drugs, such as benzodiazepines (BZ). Each receptor subunit also contains a large intracellular domain between TM3 and TM4, which is the site for various protein interactions as well as the site for various post-translational modifications that modulate receptor activity. (B) Five subunits from 7 subunit subfamilies (α,β,γ,δ,ε,θ,π) assemble to form a heteropentameric chloride-permeable channel. Despite the extensive heterogeneity of GABAA receptor subunits, the majority of GABAA receptors expressed in the brain consist of 2α, 2β, and 1γ subunit, where the γ subunit can be replaced by δ, ε or π. Binding of the neurotransmitter GABA occurs at the interface between the α and β subunits and triggers the opening of the channel, allowing the rapid influx of chloride ions. BZ-binding occurs at the interface between α(1,2,3 or 5) and γ subunits and potentiates GABA-induced chloride flux. (C) GABAA receptors composed of α(1–3) subunits together with β and γ subunits are thought to be primarily synaptically localized, whereas α5βγ receptors are located largely at extrasynaptic sites. Receptors composed of the aforementioned subunits are benzodiazepine-sensitive. In contrast, receptors composed of α(4,6)βδ are benzodiazepine-insensitive, and are localized at extrasynaptic sites.