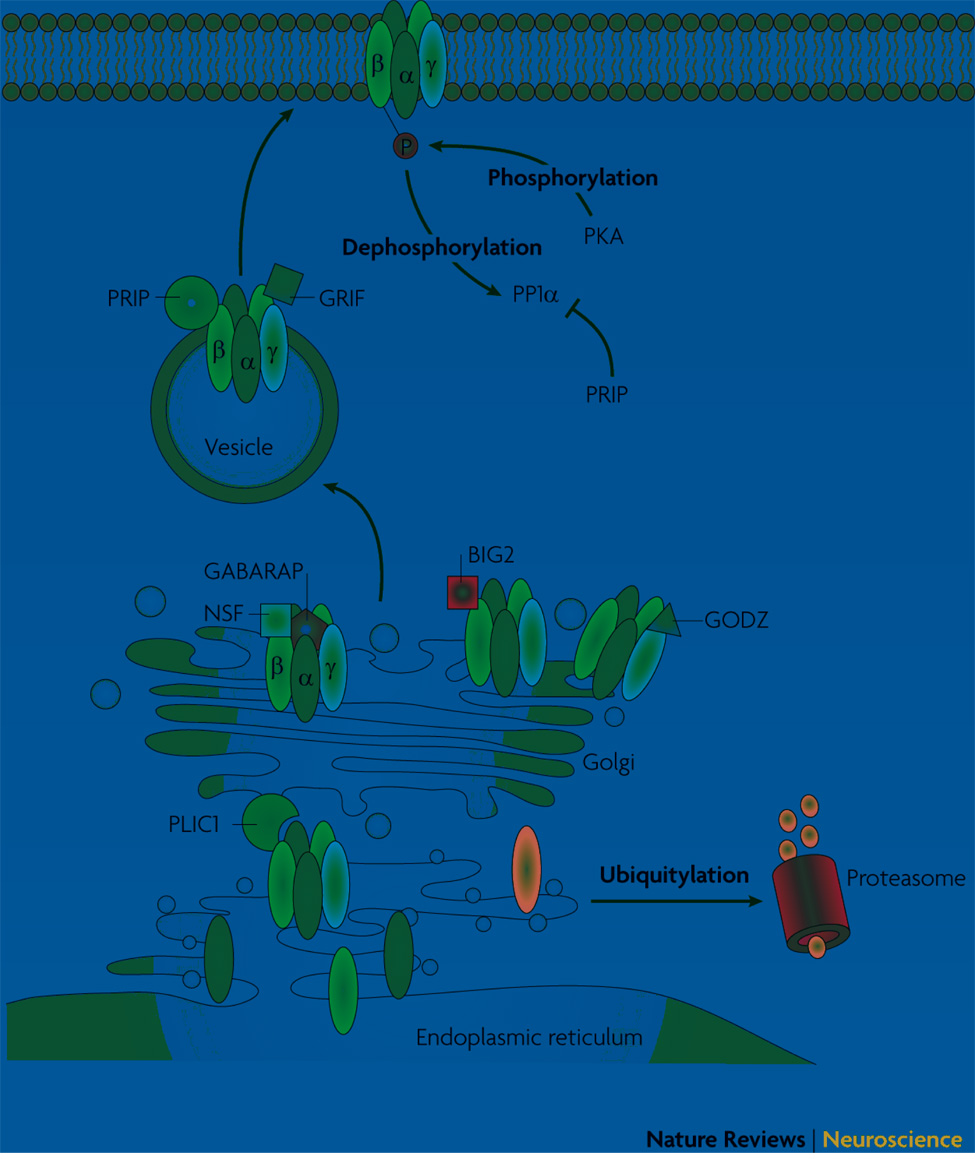
Figure 2. Trafficking of GABAA receptors.
GABAA receptor subunits are synthesized and assembled into pentameric structures in the endoplasmic reticulum (ER). This process is carefully regulated in the ER. The fate of GABAA receptor subunits can be modulated by ubiquitination and subsequent ER-associated degradation via the proteasome. Ubiquitinated GABAA receptor subunits can also be modulated via their association with Plic-1. Plic-1 facilitates GABAA receptor accumulation at the synapse by preventing the degradation of ubiquitinated GABAA receptors. Exit into the Golgi network and subsequent trafficking to the plasma membrane are also facilitiated by a number of GABAA receptor-associated proteins. GABARAP associates with the γ2 subunit of GABAA receptors and aids in the trafficking of receptors from the Golgi network to the plasma membrane. NSF and BIG2 are also localized to the Golgi network, where they bind to β subunits of GABAA receptors and modulate receptor trafficking. Palmitoylation of γ subunits occurs in the Golgi apparatus as a result of an association with the palmitoyltransferase, GODZ, and is a critical step in the delivery of GABAA receptors to the plasma membrane. GRIF proteins play a role in the trafficking of GABAA receptors to the membrane. PRIP proteins also play essential roles in the trafficking of GABAA receptors, as well as in modulating the phosphorylation state of GABAA receptors.