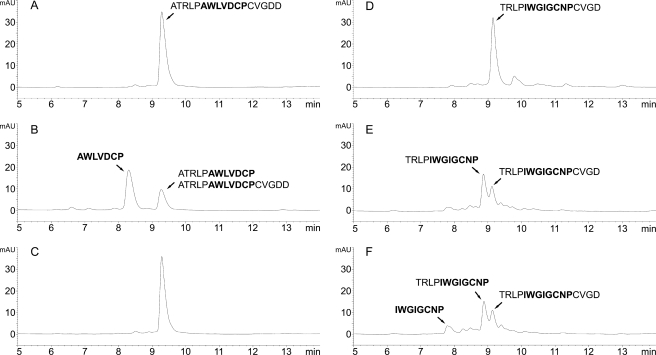
FIGURE 7.
Cleavage of phallacidin and amanitin synthetic precursor peptides. A, inactivated CaPOP1 incubated for 2 h with the 17-mer phallacidin precursor (Table 1). B, active CaPOP1 incubated for 2 h with the phallacidin precursor. The intermediate of the reaction with the phallacidin precursor could not be resolved from the uncleaved peptide by HPLC but could be unambiguously identified by MS. C, as in panel B but with the addition of the POP inhibitor Z-Pro-prolinal at 1 μm. D–F, processing of the 16-mer α-amanitin precursor (Table 1) by CaPOP1. D, boiled control. E, 1-h incubation. F, 2-h incubation. The same batch of purified enzyme was used for all of the experiments shown in this figure, which was different from the one used in the experiment shown in Fig. 4. mAU, milliabsorbance units.