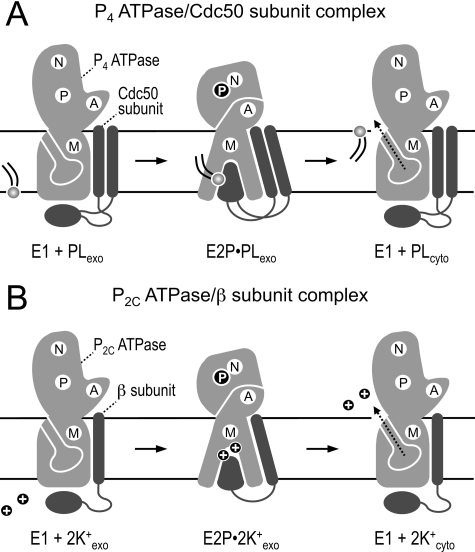
FIGURE 8.
Model of reaction cycle-dependent transporter-subunit rearrangements in P2- and P2C-ATPase complexes. A and B, schematics of the E1 and E2P forms of P4 (A) and P2C-ATPases (B) in complex with their accessory subunit (Cdc50 subunit for P4-ATPases; β subunit for P2C-ATPases). Movements of the three cytoplasmic domains (P, N, and A) and intramembrane region (M) of the ATPase are accompanied by changes in ATPase-subunit binding affinity. The E1 form, which has a relatively low affinity for the subunit, is accessible for ligands from the cytosol (unknown for P4-ATPases; Na+ or H+ for P2C-ATPases). Binding of ligand promotes phosphorylation of E1 to create E1P (not shown). During conversion of E1P to E2P, the ligands are discharged to the exoplasmic side, and counter-transported ligands (phospholipid for P4-ATPases; K+ for P2C-ATPases) can now bind. This is accompanied by a tighter association of the ATPase to the subunit, presumably involving a high affinity interaction between the ATPase and the large ectodomain of the subunit (49, 53).5 The latter may contribute to formation of the ligand-binding site or serve as a lid to close access to the ligand-binding site from the exoplasmic side. Failure to achieve this specific arrangement may block dephosphorylation of E2P, hence preventing the ATPase from continuing successfully through the remainder of the cycle. During reversion to the E1 state, counter-transported ligands are released to the cytoplasmic side. See “Discussion” for further details. PL, phospholipid; N, nucleotide-binding domain; P, phosphorylation domain; A, actuator domain; M, intramembrane domain.