Table 1.
Domino Heck–aza-Michael.[a]
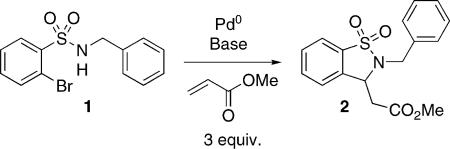 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Base | Additive | % Conv.[b] |
| 1 | Pd(OAc)2/PPh3 | Et3N | – | 34 |
| 2 | Pd(OAc)2/PPh3 | Cs2CO3 | – | 15 |
| 3 | PdCl2(PPh)3 | Cs2CO3 | – | 13 |
| 4 | Pd2(dba)3·CHCl3 | Et3N | – | 35 |
| 5 | Pd(OAc)2/PPh3 | Et3N | Bu4NCl | 91 |
| 6 | Pd2(dba)3·CHCl3 | Et3N | Bu4NCl | 95 |
| 7 | Pd(OAc)2/PPh3 | Et3N | – | 94[c] |
| 8 | Pd(OAc)2/PPh3 | Et3N | Bu4NCl | 95[d] |
Reaction conditions: 1 (0.12 mmol), Pd (2 mol-%), methyl acrylate (0.36 mmol), base (0.36 mmol) in DMF at 110 °C for 14 h.
Conversion by G.C.
Pd(OAc)2 (20 mol-%), PPh3 (40 mol-%) in microwave 110 °C for 2 h.
Pd(OAc)2 (10 mol-%), PPh3 (20 mol-%) in microwave 110 °C for 2 h.
