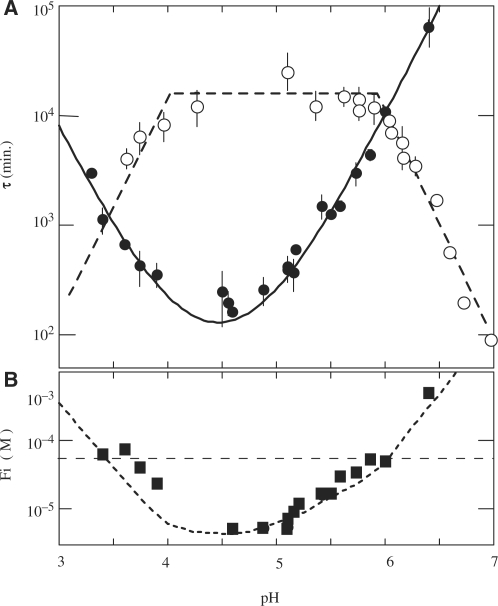
Figure 2.
Effect of pH on the formation and dissociation times and on the stability of the fully intercalated [TC3]4 tetramer. (A) [TC3]4 lifetime (open circles) and half formation time in 10−4 M TC3 solutions (black circles). The half formation time varies with pH as the power −2 of the product of the fraction of neutral cytidine, 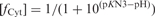 and protonated cytidine:
and protonated cytidine: 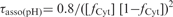 . (B) Reduced dissociation constant versus pH. The observation that Fi is close 10−4/2 (dashed line) at the temperature for which the association time measured in 10−4 M TC3 solution and the dissociation time are equal shows the consistence of the kinetics and equilibrium measurements.
. (B) Reduced dissociation constant versus pH. The observation that Fi is close 10−4/2 (dashed line) at the temperature for which the association time measured in 10−4 M TC3 solution and the dissociation time are equal shows the consistence of the kinetics and equilibrium measurements.