The Centers for Medicare & Medicaid services (CMS) proposes to change the method of reimbursement for outpatient hemodialysis such that a fixed payment bundle will cover both outpatient dialysis therapy and injectable medications. The proposal does not include an adjustment for race, although this is up for debate. We aimed to determine if African Americans, compared with whites, continue to initiate dialysis with lower hemoglobin concentrations and require higher doses of erythropoiesis stimulating agents (ESA) to achieve similar hemoglobin concentrations, as they have historically. We constructed a cohort of 12,002 ESA-naive patients older than 67 yr who initiated hemodialysis between January 1, 2006 and October 31, 2006, had Medicare as their primary payer for 2 yr preinitiation, and received erythropoietin (EPO) during the first 2 mo postinitiation. At dialysis initiation, African Americans had lower hemoglobin values than whites (9.9 ± 1.7 versus 10.3 ± 1.6 g/dl, P < 0.001). On average, after adjusting for multiple variables (but not initial hemoglobin concentration), African Americans required 11.0% more EPO than whites (P < 0.001). With initial hemoglobin included in the model, this difference was attenuated to 6.8% more EPO than whites (P < 0.001). In conclusion, if CMS implements its proposed reimbursement scheme, facilities may have a financial disincentive to treat African Americans, potentially resulting in reduced access to outpatient dialysis therapy.
The Centers for Medicare & Medicaid services (CMS) has proposed to change the method by which outpatient hemodialysis is reimbursed. Under the current schema, outpatient dialysis is billed at a fixed rate, and intravenous (IV) medications are billed separately on the basis of dose. In the current system, greater use and higher doses of IV medications result in increased revenue for outpatient dialysis facilities, potentially creating an incentive toward greater use of outpatient IV medications. Under the proposed new payment scheme, a fixed payment bundle will cover outpatient dialysis therapy and injectable medications.1 The proposal attempts to adjust for cost differences between patients by using basic case-mix information to modify the monthly capitated dialysis payment. Proposed adjusters to the base payment include age, body mass index (BMI), body surface area, and locality. The proposal as recommended by CMS does not adjust for race, but this point is currently up for debate.2
Historically, African Americans have initiated dialysis with lower hemoglobin concentrations than whites and are less likely to have received erythropoiesis stimulating agent (ESA) therapy before initiating dialysis.3 However, for given hemoglobin concentrations, outcomes for African Americans appear similar to outcomes for whites.4 Furthermore, African Americans may require higher ESA doses than whites to achieve similar hemoglobin concentrations for reasons such as intrinsic resistance, poorer dialysis, or higher weight.5
A fixed-rate reimbursement system with no adjustment for race or separate billing for medications may bias against African Americans, because they may require higher ESA doses to achieve National Kidney Foundation Kidney Disease Outcomes Quality Initiative6 recommended hemoglobin targets. We thus aimed to determine in a modern cohort if (1) African Americans continue to initiate hemodialysis with lower hemoglobin concentrations than whites, and (2) African Americans use higher ESA doses than whites after hemodialysis initiation.
RESULTS
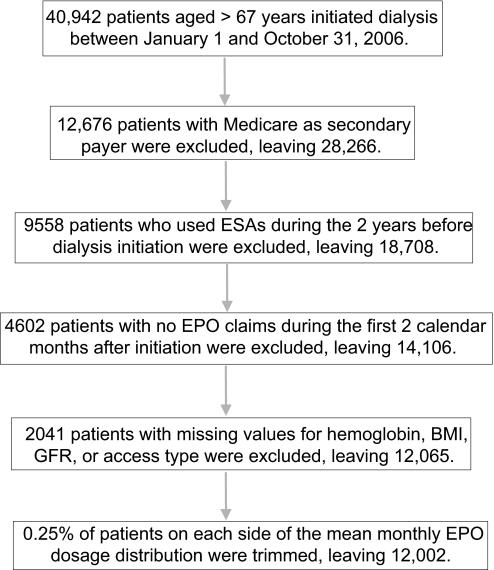
Between January 1, 2006 and October 31, 2006, 40,942 patients aged older than 67 yr initiated hemodialysis. We excluded those without Medicare as primary insurance type (n = 12,676) and those who had used ESAs during the 2 yr preinitiation (n = 9558). Of the remaining 18,708, 4602 had no ESA claims in the first 2 calendar months after initiating dialysis and were excluded. An additional 2041 were excluded because at least one of the following variables was missing from the Medical Evidence Report: hemoglobin, BMI, creatinine, or access type. Finally, because of the skewed distribution of erythropoietin (EPO) dose in the first 2 mo, we trimmed the extreme 0.25% of patients on both sides of the mean. This left 12,002 individuals in our final analysis cohort (Figure 1).
Figure 1.
Flow diagram of study selection process.
Participants included in our final cohort (n = 12,002) were generally similar to those excluded (n = 28,940) with the following exceptions: in logistic regression analysis, included participants were more likely to be older [odds ratio (OR) 1.01]; to have lower hemoglobin (OR 0.93), higher BMI (OR 1.01), and vascular access type other than fistula (OR 0.73); to have had a cerebrovascular accident/transient ischemic attack (OR 1.07) or chronic obstructive pulmonary disease (OR 1.15); to be smokers (OR 1.20), drug dependent (OR 1.34), or institutionalized (OR 1.19); and to need assistance with daily activities (1.09). Included patients were also less likely to have diabetes (OR 0.88) or cancer (OR 0.91) (Table 1).
Table 1.
Odds of being included in the final cohorta
| Variable | OR of Inclusion | P |
|---|---|---|
| Age (yr) | 1.01 | <0.001 |
| Hemoglobin (g/dl) | 0.93 | <0.001 |
| BMI (kg/m2) | 1.01 | 0.003 |
| Estimated GFR (ml/min/1.73 m2) | 1.00 | 0.1 |
| Race | ||
| white | Reference | |
| African American | 1.00 | 1.0 |
| Gender | ||
| male | Reference | |
| female | 1.02 | 0.522 |
| Access type | ||
| AV fistula | 0.73 | <0.001 |
| other access | Reference | |
| Comorbid conditions | ||
| congestive heart failure | 1.01 | 0.573 |
| ASHD | 0.98 | 0.489 |
| CVA/TIA | 1.07 | 0.048 |
| other cardiac | 1.04 | 0.210 |
| peripheral vascular disease | 1.02 | 0.622 |
| diabetes | 0.88 | <0.001 |
| COPD | 1.15 | <0.001 |
| hypertension | 0.97 | 0.281 |
| smoker | 1.20 | 0.002 |
| cancer | 0.91 | 0.014 |
| addiction or drug dependence | 1.34 | 0.024 |
| inability to ambulate or transfer | 0.98 | 0.597 |
| amputation | 0.94 | 0.382 |
| institutionalized | 1.19 | <0.001 |
| needs assistance with daily activities | 1.09 | 0.029 |
ASHD, atherosclerotic heart disease; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack.
In our final cohort (n = 12,002), compared with whites, African Americans were younger (75.7 ± 6.3 versus 77.2 ± 6.3 yr, Table 2); more likely to be women (58.9 versus 44.1%); more likely to have a graft for vascular access (6.3 versus 3.2%), previous cerebrovascular accident (17.2 versus 12.3%), diabetes (55.2 versus 46.3%), hypertension (89.0 versus 82.4%), difficulty ambulating (13.5 versus 10.4%), and an amputation (3.4 versus 2.1%); and more likely to be institutionalized (13.7 versus 12.9%) and to need assistance with daily activities (19.2% versus 16.1%). African Americans were less likely than whites to have congestive heart failure (40.7 versus 44.9%), atherosclerotic heart disease (22.1 versus 34.5%), peripheral vascular disease (13.7 versus 20.9%), chronic obstructive pulmonary disease (8.8 versus 15.7%), or cancer (9.4 versus 11.4%).
Table 2.
Patient baseline characteristicsa
| Variable | Race
|
|
|---|---|---|
| White | African American | |
| Age (yr) | 77.2 (6.3) | 75.7 (6.3) |
| Hemoglobin (g/dl) | 10.3 (1.6) | 9.9 (1.7) |
| BMI (kg/m2) | 27.3 (6.7) | 27.6 (7.6) |
| Estimated GFR (ml/min/1.73 m2) | 11.3 (5.0) | 11.4 (5.0) |
| All | 8979 (100.0) | 2432 (100.0) |
| Gender | ||
| male | 5022 (55.9) | 1000 (41.1) |
| female | 3957 (44.1) | 1432 (58.9) |
| Access type | ||
| AV fistula | 911 (10.2) | 223 (9.2) |
| graft | 291 (3.2) | 153 (6.3) |
| catheter | 7655 (85.3) | 2025 (83.3) |
| other | 122 (1.4) | 31 (1.3) |
| Comorbid conditions | ||
| congestive heart failure | 4030 (44.9) | 989 (40.7) |
| ASHD | 3101 (34.5) | 538 (22.1) |
| CVA/TIA | 1107 (12.3) | 418 (17.2) |
| other cardiac | 2158 (24.0) | 399 (16.40 |
| peripheral vascular disease | 1879 (20.9) | 333 (13.7) |
| diabetes | 4158 (46.3) | 1343 (55.2) |
| COPD | 1409 (15.7) | 213 (8.8) |
| hypertension | 7401 (82.4) | 2164 (89.0) |
| smoker | 379 (4.2) | 71 (2.9) |
| cancer | 1027 (11.4) | 229 (9.4) |
| addiction or drug dependence | 85 (1.0) | 21 (0.9) |
| inability to ambulate or transfer | 933 (10.4) | 329 (13.5) |
| amputation | 192 (2.1) | 82 (3.4) |
| institutionalized | 1160 (12.9) | 333 (13.7) |
| needs assistance with daily activities | 1449 (16.1) | 466 (19.2) |
Values for age, hemoglobin, BMI, and estimated GFR are presented as mean (SD). All other values are presented as n (column percent).
Initial Hemoglobin
At dialysis initiation, African Americans had lower hemoglobin values than whites (9.9 ± 1.7 versus 10.3 ± 1.6 g/dl, P < 0.001). However, more African Americans than whites in our cohort were women (58.9 versus 44.1%), a possible influence on the initial hemoglobin concentration. In multiple variable regression analysis, including adjustment for sex, a small difference (−0.348 g/dl, P < 0.001) remained in initial hemoglobin concentrations between African Americans and whites (Table 3).
Table 3.
Linear regression predicting initial hemoglobin concentration (n = 12,002)
| Variables | Linear Regression
|
|
|---|---|---|
| Estimates | P | |
| Age | 0.005 | 0.034 |
| BMI | −0.001 | 0.527 |
| Estimated GFR | 0.046 | <0.001 |
| Race | ||
| white | Reference | |
| African American | −0.348 | <0.001 |
| Gender | ||
| male | Reference | |
| female | −0.011 | 0.702 |
| Access type | ||
| AV fistula | Reference | |
| graft | −0.256 | 0.003 |
| catheter | −0.519 | <0.001 |
| other | −0.721 | <0.001 |
| Comorbid conditions | ||
| congestive heart failure | −0.073 | 0.019 |
| ASHD | 0.065 | 0.051 |
| CVA/TIA | 0.051 | 0.232 |
| other cardiac | 0.081 | 0.020 |
| peripheral vascular disease | 0.004 | 0.923 |
| diabetes | −0.060 | 0.044 |
| COPD | −0.044 | 0.302 |
| hypertension | 0.058 | 0.138 |
| smoker | −0.002 | 0.985 |
| cancer | −0.074 | 0.115 |
| addiction or drug dependence | −0.196 | 0.195 |
| inability to ambulate or transfer | −0.128 | 0.023 |
| amputation | −0.155 | 0.106 |
| institutionalized | −0.005 | 0.917 |
| needs assistance with daily activities | −0.098 | 0.035 |
EPO Dose
Average EPO dose after dialysis initiation was an age-adjusted 94,222 units per month. Race significantly influenced the EPO dose. On average, after adjusting for age, whites used 93,132 units of EPO and African Americans 100,659 units. In multivariable regression analysis (Table 4), with EPO dose log-transformed, and not including the initial hemoglobin concentration, African Americans continued to require significantly higher EPO units. On average, African Americans required 11.0% (P < 0.001) more EPO than whites. These results were attenuated when initial hemoglobin concentration was added to the model, but still showed African Americans using 6.8% (P < 0.001) more EPO units per month than whites.
Table 4.
Linear regression predicting EPO use per 30 outpatient days at risk
| Variables | Linear Regression Predicting Monthly EPO Dose (Units per 30 Days)
|
|||
|---|---|---|---|---|
| Initial Hemoglobin not in Model
|
Initial Hemoglobin in Model
|
|||
| Estimates | P | Estimates | P | |
| Age | −0.006 | <0.001 | −0.005 | <0.001 |
| Hemoglobin (g/dl) | −0.109 | <0.001 | ||
| BMI | 0.008 | <0.001 | 0.008 | <0.001 |
| Estimated GFR | −0.009 | <0.001 | −0.004 | 0.007 |
| Race | ||||
| white | Reference | |||
| African American | 0.104 (11.0%)a | <0.001 | 0.066 (6.8%)a | <0.001 |
| Gender | ||||
| male | Reference | |||
| women | −0.067 | <0.001 | −0.068 | <0.001 |
| Access type | ||||
| AV fistula | Reference | Reference | ||
| graft | 0.064 | 0.128 | 0.036 | 0.381 |
| catheter | −0.008 | 0.741 | −0.064 | 0.005 |
| other | −0.064 | 0.314 | −0.142 | 0.021 |
| Comorbid conditions | ||||
| congestive heart failure | <0.001 | 0.995 | −0.008 | 0.594 |
| ASHD | 0.011 | 0.487 | 0.018 | 0.246 |
| CVA/TIA | −0.042 | 0.045 | −0.036 | 0.075 |
| other cardiac | −0.017 | 0.323 | −0.008 | 0.631 |
| peripheral vascular disease | 0.010 | 0.603 | 0.010 | 0.578 |
| diabetes | 0.009 | 0.526 | 0.003 | 0.845 |
| COPD | 0.004 | 0.834 | 0.000 | 0.984 |
| hypertension | 0.024 | 0.210 | 0.030 | 0.104 |
| smoker | −0.046 | 0.211 | −0.046 | 0.198 |
| cancer | 0.060 | 0.008 | 0.052 | 0.018 |
| addiction or drug dependence | −0.053 | 0.471 | −0.075 | 0.300 |
| inability to ambulate or transfer | −0.048 | 0.079 | −0.062 | 0.020 |
| amputation | −0.050 | 0.280 | −0.067 | 0.139 |
| institutionalized | −0.034 | 0.157 | −0.034 | 0.140 |
| needs assistance with daily activities | −0.039 | 0.088 | −0.049 | 0.026 |
Represents the percent difference in ESA dose between African Americans and whites, calculated as (eparameter estimate − 1).
Stratified Analysis
Vascular access at dialysis initiation was a significant predictor of hospitalization during the first 2 mo of dialysis. Percents of patients hospitalized during the first 2 mo by vascular access type were: arteriovenous (AV) fistula, 29.2%; graft, 39.7%; catheter, 42.2% (P < 0.001). When stratified by initial vascular access type, African Americans continued to require higher doses of EPO, irrespective of vascular access type (AV fistula 7.1% more, P = 0.2; graft 14.0% more, P = 0.06; catheter 6.6% more, P < 0.001; table not shown.)
DISCUSSION
Our results in a cohort of older hemodialysis patients incident in 2006 demonstrate that despite similar health insurance and BMI, African Americans initiate hemodialysis with lower hemoglobin concentrations than whites. Additionally, in multivariable analysis, African Americans require approximately 11.0% more EPO units per month than whites per month for the first 2 mo after initiating dialysis. We chose not to include hemoglobin in our primary model, because we were interested in determining actual EPO use from a payer perspective. However, even when accounting for initial hemoglobin concentrations, African Americans still require 6.8% more EPO units per month than whites. This difference in EPO use may have policy implications, given the upcoming changes in dialysis reimbursement by Medicare.
Our observation of lower hemoglobin concentrations among African Americans than whites is similar to earlier observations.7,8 Ward et al.,9 using a cohort of dialysis patients incident between 1996 and 2004, demonstrated that hemoglobin concentration at dialysis initiation was lower for African Americans than whites, and that African Americans were less likely to have been treated with ESAs before dialysis initiation. Part of this discrepancy was related to the absence of medical insurance at dialysis initiation. Similar results have been observed using prevalent dialysis cohorts.10 Frankenfield et al.,11 using the ESRD core indicator project (a random sample of prevalent dialysis patients), indicated that despite similar body surface area values, African Americans required higher total EPO doses than whites after adjusting for weight. Specifically, African Americans used approximately 2400 more EPO units per month than whites. Despite higher doses, hematocrit values for African Americans remained lower than for whites (33.0 versus 33.2%). Also, African Americans were more likely to have hematocrit values below target (<28%) than whites (12 versus 9%).
Not all previous studies support these observations. Coladonato et al.12 also used the ESRD core indicator project and observed a similar difference in hematocrit between African Americans and whites (32.2 versus 32.6%), but no difference in EPO dose, after adjustment for predialysis weight. However, predialysis weights in their cohort were significantly higher for African Americans than whites,11 suggesting that total EPO use may have been higher among African Americans. Finally, Lacson et al.13 recently examined EPO dose differences by race in a prevalent hemodialysis cohort in the Fresenius Medical Care system. In unadjusted analysis, African Americans used approximately 12.6% more EPO than whites; however, this difference in EPO dose disappeared in multivariable analysis. The primary factors responsible for the difference in EPO dose were Kt/V, parathyroid hormone, and white blood cell count. Although differences in these factors may explain the “causal” difference in EPO dose between African Americans and whites, none of the factors are included in the new Medicare dialysis reimbursement proposal. Thus, the newly proposed dialysis bundle may provide reimbursement for African Americans that is disproportionately low compared with white dialysis patients.
Overall, these results, combined with our own, suggest that African Americans initiate dialysis with lower hemoglobin concentrations than whites, and that the difference persisted into 2006. We also observed a difference in initial hemoglobin concentrations between African Americans and whites, despite similar medical insurance types in the 2 yr before dialysis initiation. Additionally, African Americans appear to require higher ESA doses. In our cohort, despite adjusting for BMI and other factors considered in the new dialysis reimbursement bundle, African Americans used approximately 11.0% more EPO than whites, and this difference persisted after adjustment for initial hemoglobin concentration.
Numerous factors could account for the difference in hemoglobin values and ESA doses between African Americans and whites. First, in the general population, healthy African Americans have lower hemoglobin concentrations than whites, possibly reflecting an increased frequency of an alpha thalassemia deletion allele.14 African Americans on dialysis are generally younger and tend to miss more dialysis sessions than whites, potentially affecting Kt/V, parathyroid hormone,13 and overall anemia management.15 African Americans tend to have poorer dialysis than whites, also affecting anemia management.16 Finally, in a post hoc analysis of the POWER trial,17 African American nonsmokers had a diminished erythropoietic response compared with nonsmokers of other races, leading to lower hemoglobin concentrations despite controlling for ESA dose. Overall, these factors support our observation that African Americans require higher ESA doses than whites to achieve similar hemoglobin concentrations.
Although African Americans initiate hemodialysis with lower hemoglobin concentrations and require higher EPO doses during the first 2 mo of dialysis, their outcomes appear to be the same as outcomes for whites. In an incident hemodialysis population, there was no difference in time to first hospitalization between African Americans and whites in an analysis stratified by initial hemoglobin concentration.4 However, interpreting these observational results is difficult given the recent clinical trial evidence regarding ESA use and outcomes.18–20
Most dialysis facilities in the United States are for-profit entities. Currently, outpatient dialysis facilities generate revenue principally by two means: they bill for outpatient dialysis services and they bill for administration of IV medications. Given that the current reimbursement policy is tied to dose of medication administered, facilities have incentives to aggressively treat anemia using profitable, separately billable drugs such as EPO.1 The reimbursement change that CMS recently proposed to Congress, using a fixed base payment to cover the outpatient dialysis procedure and any injectable medications used during dialysis, would be “fully prospective in that facilities would keep excess payments… but would be liable for the difference if their costs exceed Medicare payments.”1 Under this proposal, the base payment would be modified based on case mix. Specific proposed adjustment factors include age (five categories), body surface area, and low BMI (<18 kg/m2). In determining how outpatient dialysis reimbursement should be changed, CMS did not consider race. If African Americans require higher ESA doses to achieve and maintain target hemoglobin concentrations, and they initiate dialysis with lower hemoglobin concentrations (also not accounted for in the reimbursement model), costs of providing dialysis care could be higher than reimbursement rates, possibly creating a disincentive to treat Africans Americans, and thus difficulties gaining access to care.
Our study is limited in several ways. Our cohort consisted of a very select population, specifically, patients aged older than 67 yr at dialysis initiation with Medicare as the primary insurance provider. As a result of our inclusion/exclusion criteria, patients included in our final cohort were likely very different from the general pool of patients aged older than 67 yr initiating dialysis. Given this difference, it is unclear how generalizable our results are to the overall hemodialysis population. However, our results are similar to previous observations in incident and prevalent dialysis populations. Also, our cohort consisted of incident hemodialysis patients in 2006. Since this time, several studies18–20 have suggested that increased hemoglobin concentrations may be associated with adverse health effects. These studies have resulted in numerous changes in anemia management, including changes in the labeled indication for ESAs, changes in reimbursement for ESA therapy, and changes in the target hemoglobin for dialysis patients. How all of these changes have influenced prescribing ESAs to hemodialysis patients is unclear. Although the changes may influence ESA doses over time, they likely do not influence initial ESA dosing, which is typically based on baseline hemoglobin and body weight.
Our results suggest that patients using catheters for dialysis access required fewer units of EPO than those using AV fistulas or grafts. This observation is in contrast to previous studies and is likely the result of our method of calculating EPO dose. We could determine only EPO doses administered in the outpatient setting. Patients with catheters were more likely to have been hospitalized during the initial 2 mo of dialysis and more likely to have been administered EPO during their inpatient stays, doses for which we could not account. We attempted to adjust for this by calculating EPO dose per outpatient day at risk. Additionally, our results did not change when we stratified by form of vascular access at baseline.
Overall, African American patients initiate hemodialysis with lower hemoglobin concentrations than whites. Additionally, African Americans require approximately 11.0% more EPO during the first few months of dialysis than whites. If this trend toward higher EPO use continues throughout the time on dialysis, and if CMS implements its currently proposed reimbursement scheme, African Americans may experience reduced access to outpatient dialysis therapy, because facilities may have a financial disincentive to accept African American patients.
CONCISE METHODS
We constructed a cohort of ESA-naïve patients who initiated hemodialysis between January 1, 2006 and October 31, 2006. Participants were required to be aged older than 67 yr at dialysis initiation and to have had Medicare as their primary source of insurance during the 2 yr before dialysis initiation. To ensure no prior ESA use at dialysis initiation, patients with Healthcare Common Procedure Coding System codes Q0136, Q0137, J0880, Q4054, and Q4055; revenue codes 0634 and 0635; and value code 68 present in their Medicare claims during the 2 yr before dialysis initiation were excluded from cohort entry. ESA therapy during the first 2 calendar months after hemodialysis initiation was also a required condition of cohort entry.
Predictor Variable: EPO Dose
Outpatient EPO use was determined from Medicare claims submitted by outpatient dialysis facilities in the first 2 calendar months after dialysis initiation. We did not include darbepoetin doses in our analysis because it was prescribed for only seven patients in our cohort during the initial 2 mo of hemodialysis. EPO doses administered during inpatient hospital stays are not billed separately to Medicare and thus could not be determined. Consequently, total outpatient EPO dose may underestimate total EPO dose used. To account for this bias, the number of days in hospital was determined for each patient on the basis of Medicare claims. Mean monthly EPO dose per outpatient day at risk was calculated for each patient by summing the total dose equivalent over a calendar month, dividing by the number of outpatient days at risk, and multiplying by 30 to obtain the total EPO dose in a calendar month (30 d).
Baseline Characteristics
Baseline characteristics at dialysis initiation were determined from the CMS ESRD Medical Evidence Report (form CMS-2728). Data extracted included demographics (age, race, sex), height, weight, comorbid conditions, primary cause of ESRD, laboratory data (hemoglobin, creatinine), and type of vascular access. BMI was determined for all patients using the Dubois formula [weight (kg)/height (m2)].
Statistics
Baseline characteristics were compared using t test for continuous variables and χ2 tests for categorical variables.
Linear regression was conducted to determine correlates of hemoglobin at dialysis initiation. Factors included in the regression model included, age, race, sex, BMI, comorbid conditions as reported on the Medical Evidence Report, vascular access type, and estimated GFR at dialysis initiation.
To determine the association between race and EPO dose during the first 2 calendar months after dialysis initiation, we constructed a linear regression model with monthly EPO dose as the predictor variable. Because it was highly skewed, monthly EPO dose was log-transformed, and the extreme was 0.25% trimmed. The skewness and kurtosis of monthly EPO dose before being log-transformed and trimmed were 1.48 and 5.21, respectively, and became −1.05 and 1.67 after transformation and trimming. As a result of this transformation, when interpreting the linear regression model, the parameter estimate for race reflects the percent difference in EPO dose between races (e.g., the percent difference in EPO dose for African Americans is calculated by the exponent of the coefficient of African Americans minus 1). Factors included in this linear regression model are age, race, sex, BMI, vascular access type, comorbid conditions, and estimated GFR. Initial hemoglobin was not included in our main model because our primary intent was to determine differences in EPO doses between races. We constructed a secondary analysis including initial hemoglobin. Also, because information on EPO units administered during inpatient hospitalizations is unavailable, we conducted a stratified analysis by type of vascular access at dialysis initiation (reported on the Medical Evidence Report), because vascular access significantly influenced the number and duration of hospitalizations during the first 2 calendar months after initiation.
For all analyses, we considered outcomes to be statistically significant if the two-sided P value was <0.05. All analyses were conducted using SAS 9.1 (Cary, NC).
DISCLOSURES
Areef Ishani consults for the Chronic Disease Research Group. Haifeng Guo, Thomas J. Arneson, Lih-Wen Mau, Suying Li, and Stephan Dunning are employed by the Chronic Disease Research Group. David T. Gilbertson and Allan J. Collins have received consulting fees from Amgen.
Supplementary Material
Acknowledgments
This study was supported by a research contract with Amgen, Inc., Thousand Oaks, California. The contract provides for the authors to have final determination of manuscript content. The authors thank Chronic Disease Research Group colleagues Shane Nygaard, BA, for manuscript preparation and Nan Booth, MSW, MPH, for manuscript editing.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Leavitt, MO. Report to Congress: A Design for a Bundled End Stage Renal Disease Prospective Payment System. Centers for Medicare and Medicaid Services. 2008. Available online at http://www.cms.hhs.gov/ESRDGeneralInformation/downloads/ESRDReportToCongress.pdf. Accessed January 27, 2009
- 2.Medicare Improvements for Patients and Providers Act of 2008 (Placed on Calendar in Senate). The Library of Congress. 2008. Available online at http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110_cong_bills&docid=f:s3101pcs.txt.pdf. Accessed January 27, 2009
- 3.Powe NR, Griffiths RI, de LG, Anderson GF, Watson AJ, Greer JW, Herbert RJ, Eggers PW, Milam RA, Whelton PK: Access to recombinant erythropoietin by Medicare-entitled dialysis patients in the first year after FDA approval. JAMA 268: 1434–1440, 1992 [PubMed] [Google Scholar]
- 4.U.S. Renal Data System: USRDS 2002 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2002, p 116
- 5.Lea JP, Norris K, Agodoa L: The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol 28: 732–743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KDOQI, National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Fried LF, Mor MK, Resnick AL, Kimmel PL, Palevsky PM, Fine MJ: Associations of race and ethnicity with anemia management among patients initiating renal replacement therapy. J Natl Med Assoc 99: 1218–1226, 2007 [PMC free article] [PubMed] [Google Scholar]
- 8.Obrador GT, Roberts T, St Peter WL, Frazier E, Pereira BJ, Collins AJ: Trends in anemia at initiation of dialysis in the United States. Kidney Int 60: 1875–1884, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Ward MM: Laboratory abnormalities at the onset of treatment of end-stage renal disease: Are there racial or socioeconomic disparities in care? Arch Intern Med 167: 2007 [DOI] [PubMed]
- 10.Volkova N, McClellan W, Soucie JM, Schoolwerth A: Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant 21: 2202–2209, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF: Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: Results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis 34: 756–759, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Coladonato JA, Frankenfield DL, Reddan DN, Klassen PS, Szczech LA, Johnson CA, Owen WF Jr: Trends in anemia management among U.S. hemodialysis patients. J Am Soc Nephrol 13: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lacson E Jr, Rogus J, Teng M, Lazarus JM, Hakim RM: The association of race with erythropoietin dose in patients on long-term hemodialysis. Am J Kidney Dis 52: 1104–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, West C: Hematologic differences between African-Americans and whites: The roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood 106: 740–745, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ifudu O, Chan E, Paul H, Mayers JD, Cohen LS, Brezsnyak WF, Herman AI, Avram MM, Friedman EA: Anemia severity and missed dialysis treatments in erythropoietin-treated hemodialysis patients. ASAIO J 42: 146–149, 1996 [PubMed] [Google Scholar]
- 16.Sehgal AR: Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA 289: 996–1000, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Jones-Burton C, Seliger SL, Brown J, Stackiewicz L, Hsu VD, Fink JC: Racial variations in erythropoietic response to epoetin alfa in chronic kidney disease and the impact of smoking. Nephrol Dial Transplant 20: 2739–2745, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Besarab A: Resolving the paradigm crisis in intravenous iron and erythropoietin management. Kidney Int Suppl S13–S18, 2006 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.