Figure 6. D1R activation enhances Akt and GSK-3β phosphorylation.
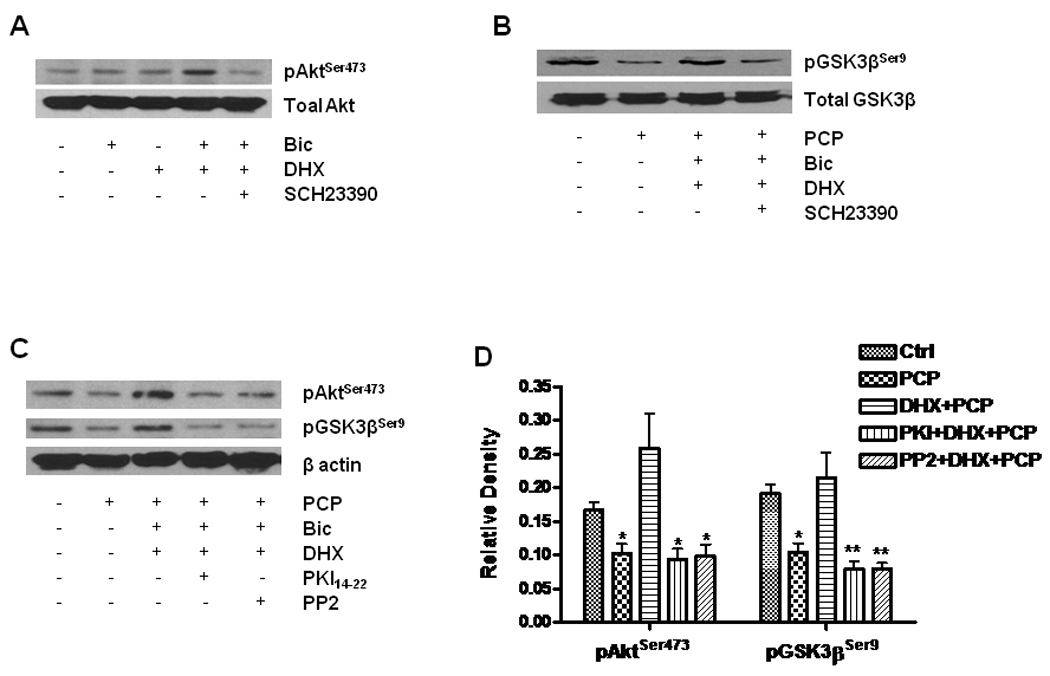
Cultured neurons at DIV14 were treated with 10 µM DHX in the presence or absence of 1 µM SCH23390 for 6h. The treated cells were lysed with RIPA buffer. Equal amounts of protein (20 µg) were loaded in each lane for WB. A. Effect of DHX on phosphorylation of Akt. Total Akt and phosphorylated Akt were determined by WB with specific antibodies against pAktSer473 and Akt in the same membrane after stripping. B. DHX reverses the reduction of pGSK-3β induced by PCP. WB shows alterations of pGSK3β and total GSK3β by probing with specific antibodies against pGSK-3βSer9 and total GSK-3β. C. The role of PKA and tyrosine kinase activation in D1R-mediated phosphorylation of Akt and GSK-3β. The cells were pretreated with 1 µM PKI14–22 or 1 µM PP2 for 30 min and then exposed to 1 µM PCP in the presence or absence of 10 µM DHX for 24h. D. Quantification of β-actin-normalized data from 3 different experiments done as shown in panel C. Values shown are mean ± SE (* p< 0.05, ** p< 0.01, one-way ANOVA, Newman-Keuls post-hoc analysis, N = 3/group).
