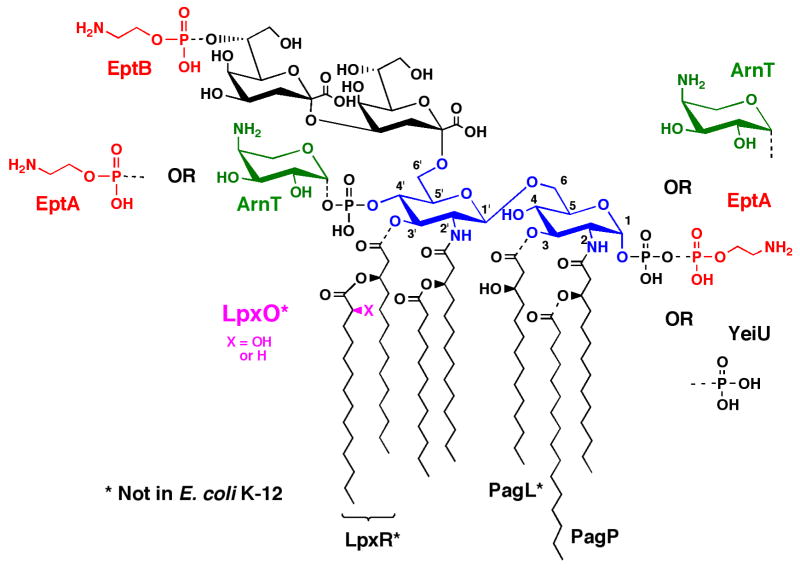
Figure 1. Regulated covalent modifications of S. typhimurium and E. coli Kdo2-lipid.
A. Covalent modifications of the lipid A phosphate groups are regulated by the PmrA/PmrB two-component signaling system (1). ArnT attaches L-Ara4N mainly to the 4′-position, whereas EptA predominantly adds pEtN mainly to the 1-position. However, the selectivity of these enzymes is reversed in the absence of Kdo (73, 74), and minor species are formed in which both positions are modified either with pEtN or with L-Ara4N (1, 50). The PagP-dependent palmitoylation (1, 17–19) and the PagL catalyzed deacylation (1, 20) of the lipid A acyl chains are under the control of the PhoP/PhoQ two-component system (1). Other lipid A modifications, such as those catalyzed by another pEtN transferase EptB (26) or the 3′-O-deacylase LpxR (1, 23), are induced by high concentrations of Ca2+. The formation of 2-hydroxymyristate on lipid A by LpxO (1, 27), observed when cells are grown in the presence of O2, is not dramatically regulated by PhoP/PhoQ under our conditions (5). The properties of the indicated enzymes responsible for these modifications are reviewed elsewhere (1). The YeiU gene, which encodes a novel phosphotransferase, was recently renamed LpxT to reflect is enzymatic function (77).