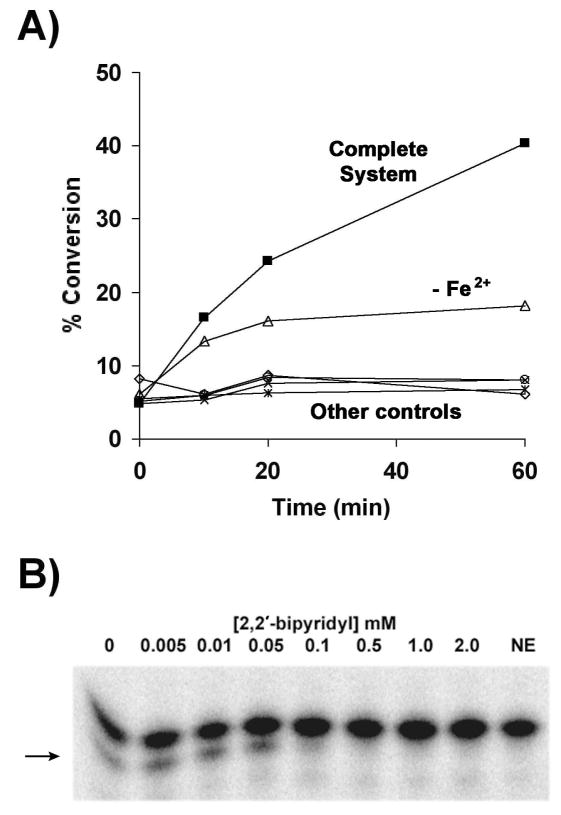
Figure 6. Dependence of LpxO activity on soluble cofactors.
A) A standard LpxO assay mixture containing 0.01 mg/ml membrane protein was prepared in which either α-ketoglutarate, ascorbate or iron was omitted. Full activity was evident only when all the cofactors were present (closed squares). Omission of α-ketoglutarate (open diamonds) or ascorbate (open circles) eliminated lipid A hydroxylation completely, as in the vector and no enzyme controls (crosses and asterisks). Omission of added Fe2+ resulted in lower activity (open triangles). B) Inhibition of LpxO activity by increasing concentrations of 2,2′-bipyridyl, an Fe2+ chelator. The standard reaction mixture containing 0.01 mg/ml protein (5 min incubation) was supplemented with the indicated concentrations of 2,2′-bipyridyl. The arrow indicates the position of the LpxO product.