Table 7. Hydrovinylation of Styrene Using Diarylphosphinite Ligandsa.
| entry | ligand | Ar | conv./y.b | sel.c | %eed |
|---|---|---|---|---|---|
| 1. | 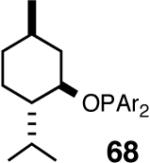 |
B | 99/86 | 86 | >5 |
| 2. | 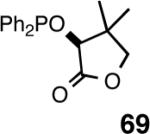 |
- | --/87 | 99 | 6 |
| 3. | 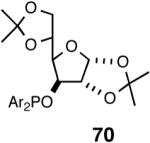 |
B | 68/68 | 99 | 29 (R) |
| 4. | 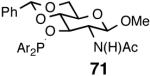 |
A | 62/62 | 99 | 32 (S) |
| 5. | 71 | B | 35/35 | 99 | 28 (S) |
| 6. | 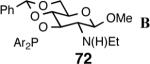 |
B | 0 | -- | -- |
| 7. | 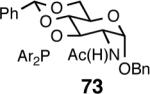 |
A | 97/93 | 96 | 9 (S) |
| 8. | 73 | B | 93/93 | 99 | 45 (S) |
| 9. | 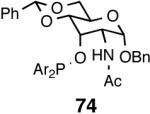 |
 |
|||
| 10. | 74 | B | 42/42 | 99 | 62 (S) |
| 11. | 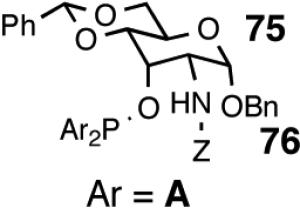 |
Z = CF3CO | 99/40 | 40e | 87 (S) |
| 12. | Z = PhCO | 99/23 | 23e | 82 (S) | |
See eq 33. A Ar = 3,5-Me2-C6H3; B Ar = 3,5-(CF3)2-C6H3.
isolated yield.
% of 3-phenyl-1-butene.
by HPLC.
conv. >99%
