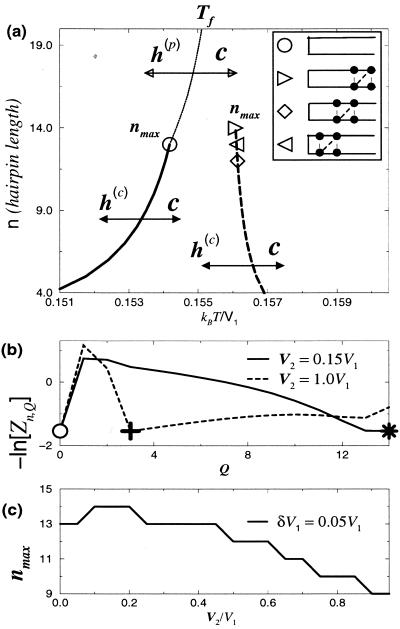
Figure 1.
(a) The typical folding temperature Tf for different polymer lengths with a two-state-like transition. The solid curve indicates Tf for peptides without heterogeneities. Above nmax (indicated by ○), the curve is replaced by a thin one where the transition is between a partially folded ensemble and the coil state. Here c represents the “coil” state, and h(c), h(p) stand for the “complete,” “partial” hairpin structure, respectively. In the presence of a hydrophobic cluster (with locations indicated in the box), Tf is increased (the dashed curve) with modified nmax (◊, central; ▹, distal-end; ◃, β-turn); the transition curves above nmax for these cases are not shown. Here σ = 1, V2 = 0.15 V1, δV1 = 0.05V1, γ = 3, and ν0 = 10−4. (b) The free energy − ln [Zn,Q] of different ensembles at Tf for n = 14 polymers with a distal-end hydrophobic cluster. Here the parameters aside from V2 are the same as in a. The symbols “○,” “∗,” and “+” stand for the coil, completely folded, and partially folded ensembles. Note that in the strong heterogeneity case, there is a highly folded ensemble (Q = 13) as an intermediate. Curves of this kind allow for the determination of nmax. (c) The dependence of nmax on the variation of V2, in the case of having a distal-end hydrophobic cluster.