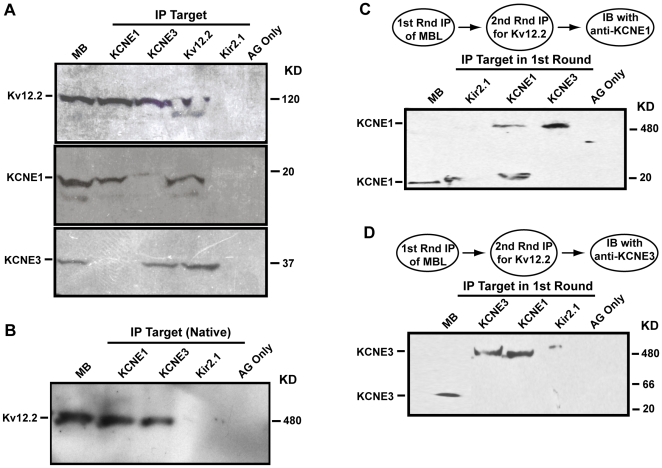
Figure 5. Kv12.2 channels simultaneously associate with KCNE1 and KCNE3 β-subunits in vivo.
(A) Mouse brain lysates (MB) were immunoprecipitated (IP) with anti-KCNE1, anti-KCNE3, anti-Kv12.2, or anti-Kir2.1 and were then subjected to SDS-PAGE and Western Blot analysis. Whole mouse brain lysate (MB) and proteins precipitated with unconjugated beads (AG only) are shown as positive and negative controls, respectively. Proteins used for IP are indicated at the top, proteins detected in Western blot (IB) are indicated at the left. Top panel, IB with anti-Kv12.2 indicates that both KCNE1 and KCNE3 interact in vivo with Kv12.2 channels. Middle panel, IB was stripped and re-probed with anti-KCNE1. KCNE1 immunoprecipitates with Kv12.2 but not KCNE3. Bottom panel, IB was then re-stripped and re-probed with anti-KCNE3. KCNE3 immunoprecipitates with Kv12.2 but does not interact with KCNE1. Controls show little or no IP of Kv12.2, KCNE1 or KCNE3 with anti-Kir2.1 or unconjugated beads. (B–D) Two-step co-immunoprecipitation assays run under native protein conditions. The Kv12.2 channel complex labeled under native conditions was ∼500 Kd, consistent with a channel tetramer. (B) Kv12.2 channels were immunoprecipitated first with anti-KCNE1 or anti-KCNE3 in these native protein conditions. Specificity was assessed using the anti-Kir2.1 antibody and unconjugated beads as negative controls. Proteins immunoprecipitated in this first IP were subjected to a second IP using anti-Kv12.2. (C) Anti-KCNE1 detected the Kv12.2 tetramer complex after the second IP regardless of whether anti-KCNE1 or anti-KCNE3 was used for the first IP. (D) Similarly, anti-KCNE3 detected the Kv12.2 complex after the second IP with anti-Kv12.2 even if anti-KCNE1 was used for the first IP. These results can be explained if KCNE1 and KCNE3 simultaneously interact with individual Kv12.2 channels. Denatured mouse brain lysate was loaded into the first lane, to confirm the established size of the respective KCNE β-subunit. Note that some KCNE1 has dissociated from the channel complex in the KCNE1 lane of (C).