Abstract
Background and Aims
Precocious flowering in apple trees is often associated with a smaller tree size. The hypothesis was tested that floral evocation in axillary buds, induced by dwarfing rootstocks, reduces the vigour of annual shoots developing from these buds compared with shoots developing from vegetative buds.
Methods
The experimental system provided a wide range of possible tree vigour using ‘Royal Gala’ scions and M.9 (dwarfing) and MM.106 (non-dwarfing) as rootstocks and interstocks. Second-year annual shoots were divided into growth units corresponding to periods (flushes) of growth namely, vegetative spur, extension growth unit, uninterrupted growth unit, floral growth unit (bourse) and extended bourse. The differences between the floral and vegetative shoots were quantified by the constituent growth units produced.
Key Results
The dwarfing influence was expressed, firstly, in reduced proportions of shoots that contained at least one extension growth unit and secondly, in reduced proportions of bicyclic shoots (containing two extension growth units) and shoots with an uninterrupted growth unit. In treatments where floral shoots were present, they were markedly less vigorous than vegetative shoots with respect to both measures. In treatments with M.9 rootstock, vegetative and floral shoots produced on average 0·52 and 0·17 extension growth units, compared with 0·77 extension growth units per shoot in the MM.106 rootstock treatment. Remarkably, the number of nodes per extension growth unit was not affected by the rootstock/interstock treatments.
Conclusions
These results showed that rootstocks/interstocks affect the type of growth units produced during the annual growth cycle, reducing the number of extension growth units, thus affecting the composition and vigour of annual shoots. This effect is particularly amplified by the transition to flowering induced by dwarfing rootstocks. The division of annual shoot into growth units will also be useful for measuring and modelling effects of age on apple tree architecture.
Key words: Apple, dwarfing, growth unit, flowering, interstock, Malus × domestica, modelling, plant architecture, polycyclic growth, shoot growth, rootstock
INTRODUCTION
Plant architecture is a relatively new and rapidly developing scientific discipline that provides powerful tools for analysis of plant structure and ontogeny (Hallé et al., 1978; Barthélémy and Caraglio, 2007). Architectural analysis is based on the hypothesis that plant structures are ‘built’ by the addition of similar constructional units (White, 1979; Barlow, 1994). The number of different construction units is relatively small and corresponds to different nested levels of organization. The basic elementary building block of plant structure is a metamer comprising a node, a leaf (or leaves), axillary bud(s) and a subtending internode (White, 1979). In a process of growth, addition of metamers builds a leafy axis. Axis extension can be continuous or rhythmic; the latter occurs in successive growth flushes (or cycles) interrupted by periods of rest, resulting in the morphologically distinct growth increments referred to as growth units (Hallé and Martin, 1968; Reffye et al., 1991). Each growth unit may have a ring of bud scales, bud scale scars and/or a zone of short internodes at its base that morphologically marks a period of rest (Barthélémy and Caraglio, 2007). In some temperate species axis extension may occur in one or more successive events during the same growing season, forming an annual shoot consisting of one or more successive growth units or growth cycles. When two or more growth units are formed during the same season they are often not identical but have distinctive features (Kozlowski, 1971). In such cases, the annual shoot level of organization is particularly useful for representation of the plant structure. It is well documented that during plant growth and branching, the repeated botanical entities such as annual shoots undergo abrupt or progressive changes in their morphological features, termed ‘differentiation’ (Gatsuk et al., 1980; Nozeran, 1984; Barthélémy et al., 1997; Barthélémy and Caraglio, 2007). While the primary aim of architectural analysis is to reveal this genetically determined plan of the plant construction, it can also be used to investigate the extent of architectural plasticity or, in other words, the extent to which an expression of the architecture can be altered by external or internal environmental factors. In this paper, architectural analysis is applied to investigate effects of rootstocks and interstocks on the structure of annual shoots, which are induced via the ‘internal’ environment of the apple tree i.e. the rootstock influence.
In apple, annual shoots develop from buds formed during the previous growing season. Annual shoots can be vegetative or floral. Vegetative annual shoots develop from vegetative buds containing about 9–11 metamers formed during the previous season (known as preformed primordia) (Rivals, 1965). Some annual shoots can also initiate and extend a number of neoformed metamers (Pratt, 1988). Floral annual shoots develop from mixed buds containing a number of preformed metamers, flower primordia in the terminal position, and two vegetative axillary structures (Pratt, 1988). Before winter the axillary structures comprise two or three primordia; at the time of budbreak in the following spring these axillary structures have five or six leaf primordia (Crabbé and Escobedo Alvarez, 1991). Following budbreak, the floral bud develops into a floral growth unit (bourse) with flowers in the terminal position, and each axillary structure can produce a ‘bourse shoot’ (Abbott, 1984; Pratt, 1988; Lauri and Terouanne, 1995). Annual shoot extension in apple may occur more than once in the same calendar year with the growth flushes (or cycles) interrupted by periods of rest (Lauri and Terouanne, 1998; Seleznyova et al., 2003).
Although dwarfing rootstocks have been widely used for many decades, the mechanisms involved and specific effects on apple tree development remain poorly understood (Atkinson and Else, 2001; Webster, 2004). The difficulty in understanding rootstock effects may be because mature trees result from successive cycles of shoot growth, flowering and fruiting that lead to cumulative and compound effects on tree structure and function (Webster, 1995). The two important traits induced by apple dwarfing rootstocks are precocity (early flowering and fruiting) and reduction in tree size (Maggs, 1955; Tustin et al., 2001; Lauri et al., 2006). It was found that on some rootstocks flowering in apple can occur along the tree trunk as early as the second year of tree growth (Seleznyova et al., 2004). These results led to the hypothesis that precocious flowering induced by dwarfing rootstocks is likely to have significant effects on the ensuing development of the apple tree form, by shifting annual shoot development from mainly monopodial (vegetative) to sympodial (floral) shoots (Seleznyova et al., 2007). These shoot types differ in structure and in the axillary bud outgrowth during the following year (Lauri and Terouanne, 1998). In addition, sympodial annual shoots subtend flowers and fruit that compete with vegetative growth for resources (Quinlan and Preston, 1971; Ferree and Palmer, 1982).
Previous studies of correlation between early flowering and smaller trees size (Lauri et al., 2006; Costes and Garcia-Villanueva, 2007) were based on retrospective analyses and considered correlations between early flowering and reduced vegetative growth using classifications based on length of annual shoots. In the current paper, the hypothesis that floral transition induced during the first year of apple tree growth has a direct effect on composition and vigour of second year annual shoots was tested. In particular, the effect of rootstock/interstock combinations on the proportions of vegetative and floral annual shoots was examined. Architectural analysis (Barthélémy, 1991; Barthélémy and Caraglio, 2007) was applied to determine the differences in the structure of these shoot types in terms of their constituent growth units and the implications of these differences on overall vigour and further development of young trees were considered. Combining these results with the previous paper by Seleznyova et al. (2003) related to rootstock/interstock effects at later stages of tree development, the sequence of structural differentiation for annual shoots was defined over a 5-year period of apple tree growth and the rootstock/interstock effects on this sequence.
MATERIALS AND METHODS
Plant material
Apple (Malus × domestica) trees were propagated in late winter of 2000. Scions made from annual shoots collected from the lower peripheral canopy of 8-year-old trees of ‘Royal Gala’ apple were grafted onto MM.106 (non-dwarf) and M.9 (dwarf) clonal rootstocks in reciprocal combinations of rootstock and interstock to provide a rootstock/interstock model system (Table 1), as previously described by Seleznyova et al. (2003). The model system comprised six treatments to produce trees of a wide vigour range but from a known restricted source of rootstock genetic material, suitable for investigating the possible mechanisms involved in rootstock dwarfing effects. Dormant, rooted stool-bed shoots of apple rootstocks were grafted to provide the six rootstock/interstock combinations. Interstock segments 30 cm long were grafted onto rootstocks at a height of 20 cm and a two-bud scion of ‘Royal Gala’ was grafted onto the top of the interstock. Treatments without interstocks were grafted with the scion at a height of 50 cm. Grafted plants were stored at 10–15 °C with roots buried in bins of moist sawdust for 1 month for callusing and were then planted in the field. The young apple trees were planted 30 cm apart along nursery rows, in a randomized complete block layout with 20 replicates of the six rootstock/interstock treatments. Rootstocks were planted precisely 15 cm deep so that the above-ground portion of the rootstock was standardized at 5 cm, with the 30-cm interstock above that. The lengths of rootstock and interstock and the depth of planting were all carefully standardized because it is known that both length of rootstock above ground and different methods of planting affect growth responses to rootstocks in apple (Wertheim, 1998). In the first year of growth, any lateral bud outgrowths on the rootstocks/interstocks were trimmed off. As soon as strong active bud growth from the scion grafts was established, each scion was trimmed so that only a single bud developed as the primary stem of the new trees. No further pruning of the scion growth was made throughout the growth period. In the spring of the second year, all flowers present were removed from the young trees shortly after petal-fall to prevent fruiting. Fourteen replicates per treatment were selected at random for studies of annual shoot development. For the remainder of the second annual growth cycle, trees were allowed to grow without any further horticultural intervention, apart from being staked in a vertical position for protection from wind damage.
Table 1.
Apple rootstock/interstock plant model system
| Treatment | Rootstock | Interstock | No. of graft unions | |
|---|---|---|---|---|
| 1 | MM.106 | MM.106 | – | 1 |
| 2 | M.9 | M.9 | – | 1 |
| 3 | MM.106/MM.106 | MM.106 | MM.106 | 2 |
| 4 | M.9/M.9 | M.9 | M.9 | 2 |
| 5 | MM.106/M.9 | MM.106 | M.9 | 2 |
| 6 | M.9/MM.106 | M.9 | MM.106 | 2 |
Structure measurement and representation
The number of trees used for data collection ranged between 11 and 14 per treatment because some of the trees were damaged by fireblight infection. At the end of the second year of growth, after leaf fall, the structure of young trees was recorded using AMAPmod methodology (Godin et al., 1997) to represent the topological structure of the young trees in the form of multiscale tree graphs with four levels of organization: plant, annual shoot, growth unit and metamer. Annual shoots were divided into growth units, each representing development during a single growth flush (Hallé and Martin, 1968; Barthélémy, 1991). Five types of growth units were distinguished.
Vegetative spur (S): vegetative growth unit with minimal internode extension and total length <2·5 cm (Boyes, 1922; Barlow, 1994),
Extension growth unit (U): vegetative growth unit with total length ≥2·5 cm. When annual shoots comprised more than one vegetative extension growth unit, the growth units were separated by a ring of bud scale scars or short internodes.
Uninterrupted growth unit (C): corresponding to vegetative extension growth during the whole growing season and which had similar length and node number to a succession of two vegetative extension growth units but without reduction (or only subtle reduction) in length of internodes separating the two. It was considered necessary to introduce this separate class of growth unit to be able to represent the difference between bicyclic annual shoots and annual shoots arising from a single extended period of uninterrupted vegetative growth. The important difference between these two types of annual shoot was the distribution of internode length along the shoot which could be an important factor affecting the pattern of axillary bud outgrowth in the following year.
Floral growth unit or bourse (F): a ‘short swollen’ preformed outgrowth bearing a terminal inflorescence (Boyes, 1922)
Extended bourse (E): comprised a fully extended preformed vegetative part, with late and often aberrant inflorescence at the terminal position.
This classification of growth units is extended from a similar previous study on older cropping apple trees by Seleznyova et al. (2003) and contains two additional types, uninterrupted growth unit and extended bourse, that were not observed in older trees.
Data analysis
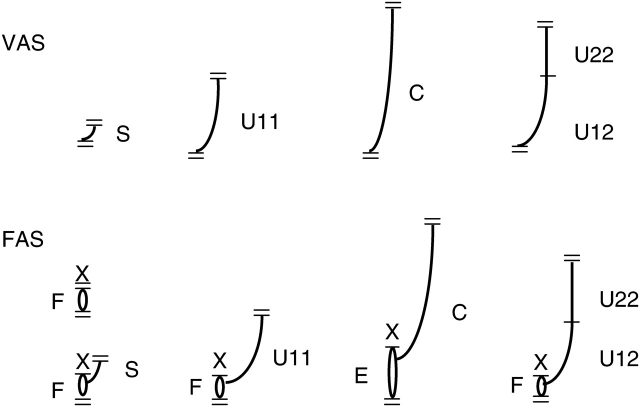
To take into account possible effects of extension growth unit position within an annual shoot, extension growth units were distinguished from shoots with one and two vegetative growth cycles: one of one (U11), first of two (U12) and second of two (U22) (Fig. 1). The following variables were extracted from the tree database using AMAPmod software and modelling language (Godin and Guédon, 1999):
Number of vegetative and floral axillary annual shoots per tree; these were further classified according to their composition: the presence and number of different types of growth units. Annual shoots that had at least one extension growth unit or a unit with uninterrupted growth were referred to as extension annual shoots.
The number of nodes per extension growth unit and per uninterrupted growth unit for vegetative and floral annual shoots.
The structure of the tree terminal bud outgrowth varied considerably even within treatments. To account for this variability, the following measures of terminal annual shoot vigour were introduced: number of growth units per annual shoot, node number per dominant axis, length of dominant axis, number of nodes of individual extension growth units according to unit type. When a dominant axis included a section of an extended bourse, the number of nodes and the length of this section were included into the calculation of the axis node number and length.
Fig. 1.
Examples of composition of vegetative (VAS) and floral (FAS) annual shoots in apple. F, Floral growth unit, bourse (represented by an oval); E, extended bourse (represented by elongated oval); S, vegetative spur; U, extension growth unit; C, uninterrupted growth unit. Index indicates growth unit type, e.g. U12, first of two growth units of annual shoot. =, Limit of annual growth; –, limit of growth unit (flush); X, termination of apical meristem. In floral annual shoots, the first growth unit is always F or E and subsequent vegetative growth can occur from an axillary meristem.
Rootstock/interstock treatment effects and shoot type effects on variables 2 and 3 were analysed using non-parametric Kruskal–Wallis tests, since these variables were not normally distributed. Negative binomial distributions were fitted to the node number distributions for monocyclic (containing one extension growth unit) and bicyclic (containing two extension growth units or uninterrupted growth units) vegetative axillary annual shoots in the treatment with MM.106 rootstock.
RESULTS
Axillary bud outgrowth
After the comparison of treatment effects, the rootstock/interstock combinations were arranged in the order from least dwarfing to the most dwarfing influence, namely: MM.106 with no interstock, MM.106/MM.106, MM.106/M.9, M.9/MM.106, M.9 with no interstock and M.9/M.9. For the first four treatments in this sequence, extension growth was progressively reduced in response to dwarfing influence (Table 2). In particular the number of vegetative extension annual shoots decreased, the number of floral annual shoots increased and, irrespective of treatment, only a small proportion of floral annual shoots developed extension growth units (Fig. 2). The treatment where M.9 was used only as an interstock on MM.106 rootstock still induced a significant reduction in the number of vegetative extension annual shoots and an increase in floral annual shoots compared with the MM.106 treatment. There were no significant differences in the number of vegetative and floral shoot types between treatments with M.9 as the rootstock, irrespective of the interstock vigour (Table 2). In addition to an overall decrease in the number of extension annual shoots with the increasing influence of dwarfing rootstock, the number of bicyclic vegetative annual shoots was also considerably reduced (Fig. 3A, top). In all treatments, only a very small proportion of floral annual shoots were bicyclic (Fig. 3A, bottom).
Table 2.
Rootstock/interstock treatment effects on the number of different axillary annual shoot types grown by young ‘Royal Gala’ apple trees in their second year of development
| Treatment | Vegetative extension shoots | Vegetative spurs | Floral shoots | Total shoots |
|---|---|---|---|---|
| MM.106 | 21·7 ± 1·0a | 18·2 ± 2·1a | 1·7 ± 0·6a | 41·6 ± 2·2a |
| MM.106/MM.106 | 18·3 ± 1·5ab | 16·4 ± 1·9ba | 2·8 ± 1·1ab | 37·8 ± 2·4a |
| MM.106/M.9 | 13·5 ± 1·9b | 16·4 ± 2·7ba | 6·2 ± 1·9b | 36·3 ± 3·0a |
| M.9/MM.106 | 6·2 ± 1·3c | 10·6 ± 2·1b | 13·4 ± 2·2c | 30·2 ± 1·7b |
| M.9 | 5·8 ± 0·7c | 11·7 ± 1·7b | 16·4 ± 1·8c | 33·8 ± 1·6bc |
| M.9/M.9 | 6·6 ± 1·0c | 9·9 ± 2·2b | 16·3 ± 1·8c | 36·6 ± 1·9ac |
Significant differences between treatments within each shoot category are indicated by different letters (P < 0·05).
Total number of shoots includes vegetative, floral and broken shoots.
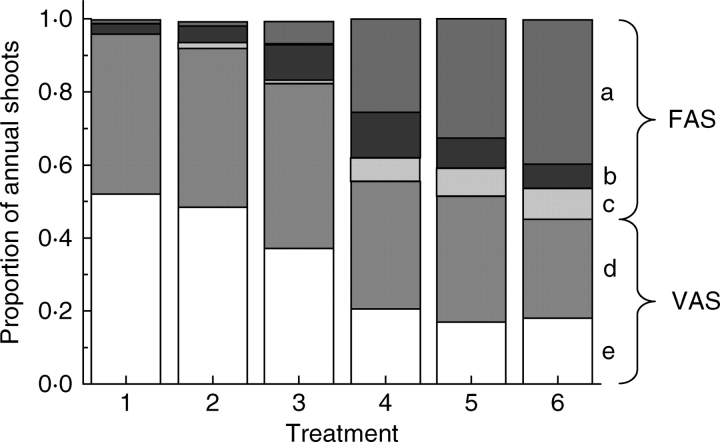
Fig. 2.
Rootstock/interstock effects on composition of ‘Royal Gala’ second-year axillary annual shoots. The treatments are arranged according to decreasing vigour (1, MM.106 with no interstock; 2, MM.106/MM.106; 3, MM.106/M.9; 4, M.9/MM.106; 5, M.9 with no interstock; 6, M.9/M.9). a, Floral annual shoot (FAS) with no vegetative extension comprising a bourse or a bourse with an axillary spur; b, floral annual shoot comprising an extended bourse or an extended bourse with an axillary spur; c, floral extension annual shoot comprising a bourse or an extended bourse with one or two extension growth units or an uninterrupted growth unit; d, vegetative annual shoot (VAS) with no extension, comprising a spur; e, vegetative extension annual shoot comprising one or two extension growth units or an uninterrupted growth unit.
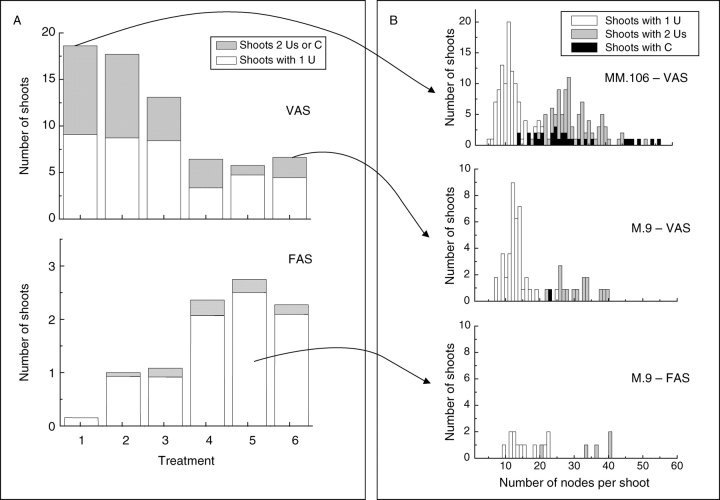
Fig. 3.
Rootstock/interstock and shoot type effects on numbers of second-year axillary annual shoots of ‘Royal Gala’ with one and two extension growth units (A), and on the shoot node number distributions (B). VAS, Vegetative annual shoots (A, top); FAS, floral annual shoots (A, bottom). The treatments are arranged according to decreasing vigour (1, MM.106 with no interstock; 2, MM.106/MM.106; 3, MM.106/M.9; 4, M.9/MM.106; 5, M.9 with no interstock; 6, M.9/M.9). The bimodal nature of the node number distribution, which is due to the presence of annual shoots with one and two extension growth units (Us) or an uninterrupted growth unit (C), is more apparent in the case of a large sample of vegetative extension annual shoots (268) in treatment with MM.106 rootstock (B, top).
The balance between the monocyclic and bicyclic annual shoots affected the shoot node number distributions (Fig. 3B). Because of the presence of these two shoot types these distributions were bimodal which was particularly apparent for treatments with MM.106 rootstock, because of a large number of extension annual shoots in these treatments. In all treatments the number of nodes per extension growth unit from monocyclic annual shoots varied between 5 and 26. For bicyclic annual shoots the total number of nodes of the combined first and the second extension growth units varied between 15 and 62. The node number distribution for a pooled population of vegetative extension annual shoots (total shoot number 268), including annual shoots with one extension growth unit, two extension growth units and uninterrupted growth units for the treatment MM.106 rootstock with no interstock, is shown in Fig. 4A. Negative binomial distributions fitted to the data for monocyclic and bicyclic annual shoots and the mixture of these distributions is also shown. The cumulative distribution function shows that the model mixture has a good fit to the data.
Fig. 4.
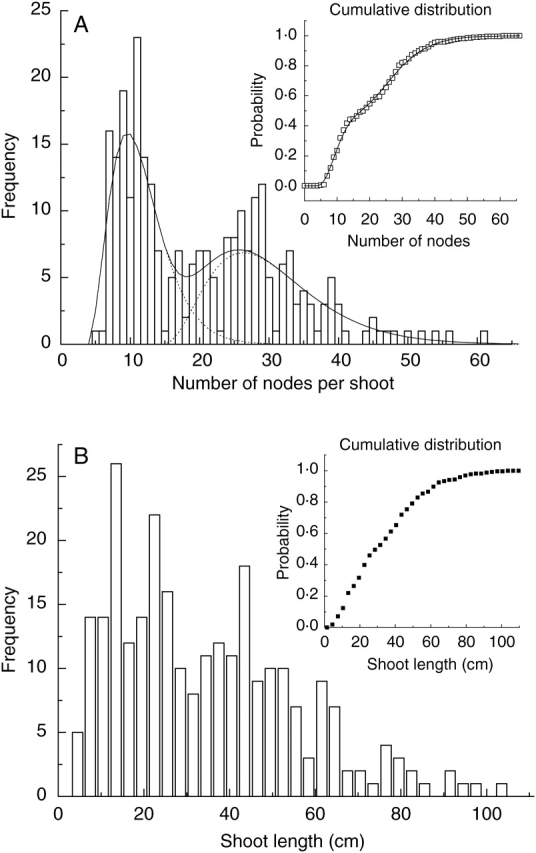
Node number (A) and shoot length (B) distributions for second-year vegetative axillary annual shoots of ‘Royal Gala’ in the treatment with MM.106 rootstock. Inserted graphs show the corresponding cumulative distribution functions. Vertical bars and symbols represent data. Dashed lines represent negative binomial distributions fitted to the data for monocyclic (mean = 11·5, SD = 4·0) and bicyclic (mean = 29·0, SD = 8·2) annual shoots. Continuous lines represent the mixture of these distributions (A, main graph) and the corresponding model for the cumulative distribution functions (A, insert graph).
For all considered treatments, the mean numbers of nodes of monocyclic and bicyclic annual shoots were similar to the treatment with MM.106 rootstock (Fig. 5A). The treatment mean number of nodes per uninterrupted growth unit varied between 27 and 34 nodes (data not shown). This variation was not related to treatment vigour and was probably due to the low number of growth units in this category (Fig. 3B). Hence the rootstock/interstock treatment affected the proportions of different growth unit categories (Fig. 3), but did not affect the number of nodes per category (Fig. 5A). To quantify the shoot type effect (vegetative/floral, monocyclic/bicyclic) on the number of nodes per first extension growth unit, the data were pooled from all treatments. The median value of nodes per extension growth unit from monocyclic vegetative shoots was 12, which was significantly smaller (P < 0·001) from the median value of 14 for the other three categories.
Fig. 5.
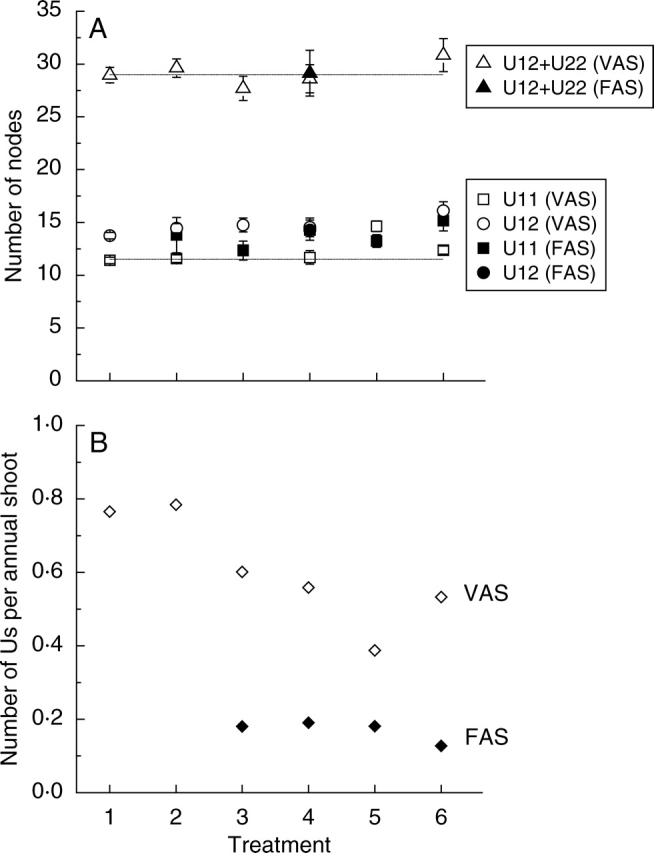
Composition of second-year axillary annual shoots of ‘Royal Gala’ apple. The treatments are arranged according to decreasing vigour (1, MM.106 with no interstock; 2, MM.106/MM.106; 3, MM.106/M.9; 4, M.9/MM.106; 5, M.9 with no interstock; 6, M.9/M.9) (A) Mean number of nodes per extension growth unit (U) of vegetative (VAS) and floral (FAS) annual shoots. Indices represent the position of a unit within an annual shoot, e.g. U12 is the first unit of two. The total mean number of nodes for two extension growth units (U12 + U22) of bicyclic annual shoots is also shown. For comparison, the mean values of negative binomial distributions fitted to the data for monocyclic and bicyclic vegetative annual shoots in treatment with MM.106 rootstock are shown by dashed lines. (B) Mean number of extension growth units per vegetative (VAS) and floral (FAS) annual shoot. For each shoot type, the treatment mean values were calculated by summing all extension growth units and dividing this sum by the total number of annual shoots in the treatment. Because the number of nodes per extension growth unit was independent of treatment or shoot type, this gave a simple measure of outgrowth from vegetative and mixed buds within each treatment.
The length of monocyclic annual shoots varied between 5 cm and 80 cm while the length of bicyclic annual shoots varied between 18 cm and 102 cm. Shoot length distribution was also bimodal (Fig. 4B); however, the shape of this distribution was slightly different from the corresponding node number distribution (Fig. 4A). The presence of two modes was more apparent in the distribution of the number of nodes per annual shoot than of annual shoot length.
Similarity in the number of nodes of extension growth units from both monocyclic and bicyclic floral and vegetative annual shoots and the independence of these numbers from the rootstock/interstock treatments allowed a simple measure of the vigour of axillary bud outgrowth to be introduced as a mean number of extension growth units per annual shoot (Fig. 5B). The number of extension growth units per vegetative annual shoot was reduced in treatments where M.9 was used as a rootstock or interstock compared with the treatments using MM.106/MM.106 and MM.106. Mean numbers of extension growth units per vegetative annual shoot for these two groups were 0·52 and 0·77, respectively. The number of extension growth units for floral annual shoots were very similar in those treatments where M.9 was used as a rootstock or interstock (Fig. 5B), and the mean value for this group was only 0·17. These data clearly indicated that the balance between vegetative annual shoots and floral annual shoots critically affected the overall tree vigour during the second year of growth.
Terminal bud outgrowth
During the first year, growth of the primary plant stems (tree trunks) was not affected by the rootstock/interstock treatment (Table 3, last column). Trunks developed as uninterrupted growth units comprised between 50 and 55 nodes. During the second year, outgrowth from terminal buds was also very vigorous, frequently with more than one bourse shoot in the case of floral annual shoots and with one or more axillary branches in the case of vegetative annual shoots (Fig. 6). Terminal annual shoots were vegetative in all trees of the most vigorous treatments (Treatments 1 and 3 in Table 1) and floral in the least vigorous treatments (Treatments 2 and 4 in Table 1). In the intermediate vigour treatments both shoot types were present. The first growth unit in floral annual shoots was most often an extended bourse that was similar to preformed extension growth units but which terminated in a late flower (data not shown). The total number of growth units per annual shoot was between four and five and was not affected by the rootstock treatments (Table 3). The node numbers per extension growth unit and per uninterrupted growth unit from terminal shoots were also unaffected by the rootstock treatment. Each terminal annual shoot had a vigorous dominant axis which contained an uninterrupted growth unit or two extension growth units. The number of nodes per dominant axis decreased in response to dwarfing influence (Table 3). The results were less clear for the length of the dominant axis. While in the treatment M.9 with no interstock, axis length was significantly lower than in treatment MM.106 with no interstock, there was a large overlap in the axis length between intermediate treatments. The composition of annual shoots in intermediate treatments was highly variable, which affected axis length. Within each treatment the number of nodes per dominant axis produced in the second year of growth was less than in the first year; this difference was significant for all treatments apart from treatment MM.106 (Table 3).
Table 3.
Rootstock/interstock treatment effects on the structure of the terminal bud outgrowths of young ‘Royal Gala’ apple trees in their second year of development
| No. of nodes per dominant axis |
Length of dominant axis (cm) |
|||||
|---|---|---|---|---|---|---|
| Treatment | Mean | Median | Mean | Median | Median no. of GUs | No. of nodes in year 1 |
| MM.106 | 49·5 ± 1·6 | 49aA | 98·9 ± 3·3 | 98a | 4a | 53A |
| MM.106/MM.106 | 48·3 ± 1·6 | 48·5aB | 94·2 ± 5·6 | 97·5a | 4·5a | 55A |
| MM.106/M.9 | 41·9 ± 4·0 | 48aB | 77·2 ± 10·2 | 85·5ab | 5a | 54A |
| M.9/MM.106 | 43·8 ± 3·2 | 44bA | 87·5 ± 8·0 | 93ab | 5a | 50·5A |
| M.9 | 37·0 ± 4·3 | 43·5bB | 80·4 ± 6·7 | 86ab | 4·5a | 53A |
| M.9/M.9 | 38·0 ± 2·6 | 40bB | 71·2 ± 6·2 | 71b | 4a | 53A |
For comparison, the median number of nodes per tree trunk at the end of the first year is shown in the last column.
Treatments/years were compared using the Kruskal–Wallis test. Significant differences between treatments within each shoot category are indicated by different lower-case letters (P < 0·01). There were no differences between the treatments in the number of nodes per tree trunk at the end of the first year and in the number of growth units (GUs) per terminal shoot in the second year. Within the treatment, differences between the number of nodes developed by a dominant axis during the first and the second year of growth are shown by different upper-case letters (P < 0·01).
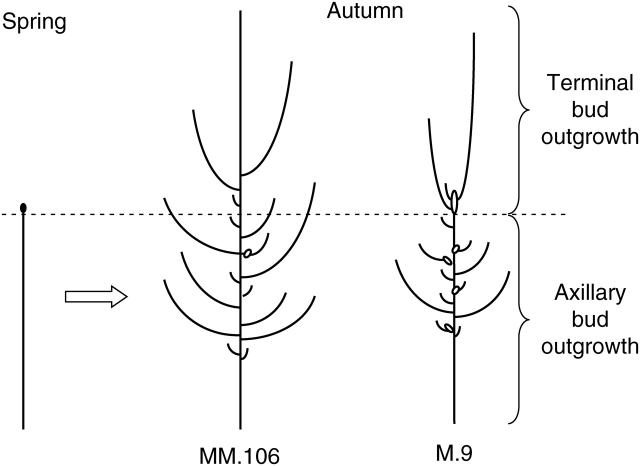
Fig. 6.
Schematic representation of rootstock effects on structural development of young ‘Royal Gala’ apple trees. Second-year outgrowths from axillary and terminal buds are separated by a dashed line. Ovals represent bourses; lines represent vegetative growth units. Boundaries between consecutive growth units are not shown. The drawing does not show the exact measured values but rather represents the conclusion that the trees on dwarfing rootstocks developed fewer extension axillary annual shoots, very few of which had two extension growth units, while the outgrowth from the terminal buds was not so much affected.
DISCUSSION
The present study demonstrated that the dwarfing rootstock and interstock had a profound effect on structural development and vigour of ‘Royal Gala’ apple trees during the second year of growth. This effect was 2-fold and was expressed, firstly, in reduced vigour of vegetative axillary annual shoots and, secondly, by replacement of vegetative axillary annual shoots with even less vigorous floral axillary annual shoots. This resulted in smaller numbers of annual shoots which contained extension growth units, in particular, of bicyclic annual shoots developed along the trunks of trees in dwarfing treatments. Costes and Garcia-Villanueva (2007) who followed 5 years of growth of two apple cultivars, either own-rooted or grafted on M.9 rootstock, found no differences in growth of floral and vegetative annual shoots and no root system effect on the number of long axes (shoots) developed in the second year. Possible explanations of the discrepancies between the two studies could be in the differences between experimental systems, methods and environmental conditions. Lauri et al. (2006) had shown that M.9 rootstock reduced mean length of second-year axillary annual shoots in apple trees. The finding of the present study was that this reduction in mean length resulted from a reduction in numbers of annual shoots with two extension growth units or uninterrupted growth rather than from an even reduction in length of all annual shoots across the population. The present study also showed that dwarfing rootstock/interstock treatments reduced not only canopy size but also the density of branches within the canopies, so that trees in dwarfing treatments had smaller and more open canopies with large vertical gaps between long branches (Fig. 7). This is likely to have an impact on future tree physiology and, in particular, on leaf area density, light distribution within the canopies and light use efficiency.
Fig. 7.

Examples of ‘Royal Gala’ apple trees from treatments with M.9 (left) and MM.106 (right) rootstocks and no interstocks at the end of the third year of growth. The photographs of the two trees were taken from the same distance. The arrows show limits of the first year of growth. The photographs clearly show the higher number of long branches developed from the first year section of the trunk for treatment with MM.106 rootstock (right). Hence the overall shape of the tree and the branch density within the canopy envelope were affected by the rootstock treatments.
The overall rootstock effect, including effects on the number of extension annual shoots and their cyclicity, could be measured by the mean number of extension growth units/cycles per axillary annual shoot. In treatments with M.9 rootstock or interstock, there were 0·17 and 0·52 extension growth units per floral and vegetative annual shoot, respectively, compared with 0·77 extension growth units per vegetative annual shoot in the treatment with MM.106 rootstock. These numbers were multiplied by the number of nodes per growth unit (which was approximately the same for all shoot types and treatments) to give numbers of extended metamers developed by floral and vegetative annual shoots, which in turn determined the numbers of axillary annual shoots produced in the following year by each shoot type (Seleznyova et al., 2003). These results confirmed our hypotheses and clearly demonstrated how floral transition induced by dwarfing rootstocks and interstocks in the first year of apple tree growth, reduced the number of extension annual shoots developed during the second year and during following years. An interstock of M.9 (treatment MM.106/M.9) induced the same type of effects on axillary annual shoots as M.9 rootstock, which implied that the dwarfing mechanism was not necessarily entirely dependent on the root function of the rootstock.
In all treatments, the terminal annual shoots were more vigorous than the axillary annual shoots, in which the dominant axes displayed uninterrupted or polycyclic growth. The M.9 rootstock reduced the number of nodes compared with MM.106 rootstock, but the difference was not more than 20 %. The rootstock effect was less clear for the length of the dominant axis because of the variability of the axis composition. Given the same total number of nodes, an axis consisting of a single uninterrupted growth unit was longer than an axis consisting of a section of extended bourse and an uninterrupted growth unit, because of the shorter internodes of the extended bourse.
The current results complemented a previous study of rootstock/interstock effects on the structure of 3-year-old branches from 5-year-old trees, using the same experimental design (Seleznyova et al., 2003). That study found that the effects of ageing, across all treatments, were expressed as an increase in the proportion of floral annual shoots and the decrease in number of extension growth units per annual shoot. The effect of ageing was similar to the dwarfing effect presented in this paper. Remarkably, in the fifth year of tree growth (Seleznyova et al., 2003), the number of nodes per extension growth unit in treatments with MM.106 rootstock was similar to the values obtained in the current study for the second year of growth, indicating a robust pattern in extension growth unit dynamics. In the fifth year, extension growth units in treatments with M.9 rootstock had fewer nodes, indicating reduction in neoformation (Seleznyova et al., 2003).
Based on these combined results, we propose a discrete sequence of qualitatively different vigour states (sequence of differentiation) for apple annual shoots that determine their final vegetative structure: annual shoot with uninterrupted extension growth; polycyclic annual shoot; monocyclic annual shoot; and non-extending annual shoot (a spur). These states are morphologically different and can be identified during the data collection and the corresponding node number distributions can be modelled using canonical distributions. These states can be used not only to quantify differences in vigour between floral and vegetative annual shoots and the effects of rootstocks but also the effects of the tree ageing on shoot growth known as ‘morphogenetic gradients’ [see a review of this concept in Barthélémy and Caraglio (2007) and references therein]. Morphogenetic gradients in apple trees were first studied by Costes et al. (2003), who followed 6 years of architectural development of ‘Fuji’ and ‘Braeburn’ grafted on M.9 type rootstock, using two trees per cultivar. While in the current paper, classification of annual shoots as monocyclic and bicyclic was based on rhythmicity of apple shoot growth where extension growth units corresponded to single flushes, Costes et al. (2003) summed successive growth flushes into annual growth units (notated ‘GUs’) classified as long (with length >20 cm), medium (with length 5–20 cm) and short (with length <5 cm). The effects of tree age on vigour of annual shoots were quantified by probabilities of different GUs according to their length (short, medium and long) and the mean number of nodes per (annual) GU was modelled by exponential functions of tree age. The current study complements these results and shows that the node number distributions for annual extension growth in apple are bimodal with the negative binomial modes corresponding to monocyclic and bicyclic annual shoots. Current and previous results suggest that the development of a growth unit, corresponding to a single flush of growth, is robust with respect to rootstock/interstock effects and tree age. Also, the use of the growth unit (flush) scale facilitates relating shoot morphology with apple tree age. For example, the growth of young trees and, especially, of the tree trunks is characterized by high proportions of annual shoots with uninterrupted or bicyclic growth, while older trees are characterized by the high proportions of monocyclic shoots and annual shoots with no vegetative extension (spurs). Hence, as the apple tree ages, the annual shoots progress along the sequence of differentiation towards less vigorous states. The node number distribution parameters can be adjusted to take into account the reduction in neoformation within growth units with tree age. Lauri et al. (2006) interpreted the dwarfing mechanism as ‘a faster physiological aging’ related to reduction in length of the second-year axillary shoots. The current results elaborated on this interpretation and indicated that the transition to flowering in the apical meristems of some annual shoots induced by the dwarfing influence resulted in less vigorous states of these shoots, thus contributing to a faster physiological aging of apple trees.
In conclusion, the sequence of discrete annual shoot vigour states proposed here for apple trees provides a basis for measuring and modelling effects of rootstocks, interstocks, shoot type (floral/vegetative), position within the tree, and tree age on annual shoot populations by the proportions of shoots in different states. These definitions are compatible with existing general methods and software for modelling plant architecture based on L-systems (Prusinkiewicz, 2004; Godin et al., 2005) and AmapSim (Barczi et al., 2008).
Defining and understanding the architectural traits of the first expression of rootstock-induced dwarfing during the growth of young trees provide new methods for identifying dwarfing rootstocks within the first 1–2 years from grafting. These traits could enable more efficient, faster breeding and evaluation of improved dwarfing apple rootstocks and newly germinated progeny in rootstock breeding families.
ACKNOWLEDGEMENTS
We thank Evelyne Costes for help with data analysis and John Palmer and Michael Clearwater for insightful comments on the manuscript. This study was funded by NZ Foundation for Research Science and Technology, Contract No. C06X0205.
LITERATURE CITED
- Abbott DL. The apple tree: physiology and management. London: Grower Books; 1984. [Google Scholar]
- Atkinson CJ, Else MA. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree. 2001;34:46–49. [Google Scholar]
- Barlow P. From cell to system: repetitive units of growth in the developments of roots and shoots. In: Iqbal M, editor. Growth patterns in vascular plants. Hong Kong: Dioscorides Press; 1994. pp. 19–58. [Google Scholar]
- Barczi JF, Rey H, Caraglio Y, De Reffye P, Barthelemy D, Dong QX, et al. AmapSim: a structural whole-plant simulator based on botanical knowledge and designed to host external functional models. Annals of Botany. 2008;101 doi: 10.1093/aob/mcm194. (in press). doi: 10·1093/aob/mcm194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy D. Levels of organization and repetition phenomena in seed plants. Acta Biotheoretica. 1991;39:309–323. [Google Scholar]
- Barthélémy D, Caraglio Y. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany. 2007;99:375–407. doi: 10.1093/aob/mcl260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy D, Caraglio Y, Costes E. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. Modelisation et simulation de l'architecture des végétaux. Versailles, France: INRA; 1997. pp. 89–136. [Google Scholar]
- Boyes D. Notes on the characters of apple tree shoots. The Journal of Pomology and Horticultural Science. 1922;3:36–46. [Google Scholar]
- Costes E, Garcia-Villanueva E. Clarifying the effects of dwarfing rootstock on vegetative and reproductive growth during tree development: a study on apple trees. Annals of Botany. 2007;100:347–357. doi: 10.1093/aob/mcm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes E, Sinoquet H, Kelner JJ, Godin C. Exploring within-tree architectural development of two apple tree cultivars over 6 years. Annals of Botany. 2003;91:91–104. doi: 10.1093/aob/mcg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé J, Escobedo Alvarez J. Activités méristématiques et cadre temporel assurant la transformations florale des bourgeons chez le pommier (Malus domestica Borkh., cv. Golden Delicious) ‘L'abre- Biologie et développment’ Naturalia Monspeliensia. 1991;7:369–380. [Google Scholar]
- Ferree DC, Palmer JW. Effect of spur defoliation and ringing during bloom on fruiting, fruit mineral level and net photosynthesis of ‘Golden Delicious’ apple. Journal of American Society for Horticultural Science. 1982;107:1182–1186. [Google Scholar]
- Gatsuk LE, Smirnova OV, Vorontzova LI, Zaugolnova LB, Zhukova LA. Age states of plants of various growth forms: a review. Journal of Ecology. 1980;68:675–696. [Google Scholar]
- Godin C, Guédon Y. AMAPmod introduction and reference manual. CIRAD: Marie-Hélène Lafond; 1999. Version 1·2. [Google Scholar]
- Godin C, Guédon Y, Costes E, Caraglio Y. Measuring and analysing plants with AMAPmod software. In: Michalewicz MT, editor. Advances in computational life sciences. Collingwood, Victoria: CSIRO Publishing; 1997. pp. 53–84. [Google Scholar]
- Godin C, Costes E, Sinoquet H. Plant architecture modelling – virtual plants and complex systems. In: Turnbull C, editor. Plant architecture and its manipulation. Oxford: Blackwell Publishing; 2005. pp. 238–287. [Google Scholar]
- Hallé F, Martin R. Etude de la croissance rythmique chez l'Hévéa (Hevea brasiliensis Müll.-Arg. Euphorbiacées-Crotonoïdées) Adansonia. 1968;8:457–503. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests. Berlin: Springer-Verlag; 1978. [Google Scholar]
- Kozlowski TT. Growth and development in trees. Vol. 1. Seed germination, ontogeny and shoot growth. Vol. 2. Cambial growth, root growth and reproductive growth. New York, NY: Academic Press; 1971. [Google Scholar]
- Lauri PE, Terouanne E. Analysis of the primary growth of apple tree branches (Malus × domestica Borkh) during one vegetation season. Canadian Journal of Botany. 1995;73:1471–1489. [Google Scholar]
- Lauri PE, Terouanne E. The influence of shoot growth on the pattern of axillary development on the long shoots of young apple trees (Malus domestica Borkh.) International Journal of Plant Sciences. 1998;159:283–296. [Google Scholar]
- Lauri PE, Maguylo K, Trottier C. Architecture and size relations: an essay on the apple (Malus × domestica, Rosaceae) tree. American Journal of Botany. 2006;93:357–368. doi: 10.3732/ajb.93.3.357. [DOI] [PubMed] [Google Scholar]
- Maggs DH. The inception of flowering in some apple rootstock varieties. Journal of Horticultural Science. 1955;30:234–241. [Google Scholar]
- Nozeran R. Integration of organismal development. In: Barlow PW, Carr DJ, editors. Positional controls in plant development. Cambridge: Cambridge University Press; 1984. pp. 375–401. [Google Scholar]
- Pratt C. Apple flower and fruit: morphology and anatomy. Horticultural Reviews. 1988;10:273–308. [Google Scholar]
- Prusinkiewicz P. Art and science for life: designing and growing virtual plants with L-systems. Acta Horticulturae. 2004;630:15–28. [Google Scholar]
- Quinlan JD, Preston AP. The influence of shoot competition on fruit retention and cropping of apple trees. Journal of Horticultural Science. 1971;46:525–534. [Google Scholar]
- Reffye de P, Elguero E, Costes E. Growth units construction in trees: a stochastic approach. Acta Biotheoretica. 1991;39:325–342. [Google Scholar]
- Rivals P. Essai sur la croissance des arbres et sur leurs systèmes de floraison. Journal d'Agriculture Tropicale et de Botanique Appliquée. 1965;12:655–686. [Google Scholar]
- Seleznyova AN, Thorp TG, White M, Tustin S, Costes E. Application of architectural analysis and AMAPmod methodology to study dwarfing phenomenon: the branch structure of ‘Royal Gala’ apple grafted on dwarfing and non-dwarfing rootstock/interstock combinations. Annals of Botany. 2003;91:665–672. doi: 10.1093/aob/mcg072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleznyova AN, White M, Tustin S, Costes E. Application of Markovian models to study rootstock/interstock effects on flowering of young apple trees. In: Godin, et al., editors. Proceedings of the 4th International Workshop on Functional-Structural Plant Models; June 7–11; Montpelier, France. 2004. pp. 311–314. [Google Scholar]
- Seleznyova AN, Tustin S, White M, Costes E. Analysis of the earliest-observed expression of dwarfing rootstock effects on young apple trees, using application of Markovian models. Acta Horticulturae. 2007;732:79–84. [Google Scholar]
- Tustin DS, Cashmore WM, Bensley RB. Pomological and physiological characteristics of Slender Pyramid central leader apple (Malus domestica) planting systems grown on intermediate vigour, semi-dwarfing and dwarfing rootstocks. New Zealand Journal of Crop and Horticultural Science. 2001;29:195–208. [Google Scholar]
- Webster AD. Rootstock and interstock effects on deciduous fruit tree vigour, precocity and yield productivity. New Zealand Journal of Crop and Horticultural Science. 1995;23:373–382. [Google Scholar]
- Webster AD. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Horticulturae. 2004;658:29–40. [Google Scholar]
- Wertheim SJ. Rootstock guide: apple, pear, cherry, European plum. The Netherlands: 1998. Publication No. 25, Fruit Research Station, Wilhelminadorp. [Google Scholar]
- White J. The plant as a metapopulation. Annual Review of Ecological Systems. 1979;10:109–145. [Google Scholar]