Abstract
Background and Aims
The cost of reproduction in dioecious plants is often female-biased. However, several studies have reported no difference in costs of reproduction between the sexes. In this study, the relative reproductive allocation and costs at the shoot and whole-plant levels were examined in woody dioecious Rhus javanica and R. trichocarpa, in order to examine differences between types of phenophase (i.e. physiological stage of development).
Methods
Male and female Rhus javanica and R. trichocarpa were sampled and the reproductive and vegetative allocation of the shoot were estimated by harvesting reproductive current-year shoots during flowering and fruiting. Measurements were made of the number of reproductive and total current-year shoots per whole plant, and of the basal area increment (BAI). The numbers of reproductive and total current-year shoots per 1-year-old shoot were counted in order to examine the costs in the following year at the shoot level.
Key Results
A female-biased annual reproductive allocation was found; however, the ratio of reproductive current-year shoots per tree and the BAI did not differ between sexes in Rhus javanica and R. trichocarpa. The percentage of 1-year-old shoots with at least one reproductive current-year shoot was significantly male-biased in R. trichocarpa, but not in R. javanica, indicating that there was a relative cost at the shoot level only in R. trichocarpa. The female-biased leaf mass per shoot, an indicator of compensation for costs, was only found in R. javanica.
Conclusions
Relative reproductive costs at the shoot level were detected in Rhus trichocarpa, which has simultaneous leafing and flowering, but not in R. javanica, which has leafing followed by flowering. However, the costs for the whole-plant level were diminished in both species. The results suggest that the phenophase type may produce the different costs for R. javanica and R. trichocarpa through the development of a compensation mechanism.
Key words: Modularity, phenology, reproductive allocation, reproductive cost, Rhus javanica, Rhus trichocarpa
INTRODUCTION
Plants partition their limited resources among functions such as growth, maintenance and reproduction (e.g. Willson, 1983; Bazzaz et al., 1987); when a plant increases resource allocation to reproduction from its limited reserves, the allocation to the other functions is reduced. Due to this trade-off, differences in reproductive allocation are believed to result in relative differences in life history traits (e.g. stem growth, flower and fruit production in the following year). Studies have focused on this trade-off between reproductive allocation and life-history traits (e.g. Delph, 1999), because the trade-off may reflect the balance between fecundity and survival probability (e.g. Iwasa and Cohen, 1989; Klinkhamer et al., 1997; de Jong and Klinkhamer, 2005).
In dioecious plants, in which female and male reproductive functions occur on different plants, more resources are typically invested in reproduction in female plants than in male plants (Lloyd and Webb, 1977; Obeso, 1997). Many authors have reported sexual dimorphisms in life history traits, such as reduced vegetative growth (Allen and Antos, 1993; Cipollini and Whigham, 1994; Rocheleau and Houle, 2001; Bañuelos and Obeso, 2004), smaller plant size (Hoffmann and Alliende, 1984; Popp and Reinartz, 1988) and less frequent flowering (Ågren, 1988; Oyama, 1990; Cipollini and Stiles, 1991) in females than in males. It has been proposed that sexual dimorphisms in life history traits (e.g. vegetative growth, plant size and reproduction in the following years) indicate the relative reproductive costs resulting from different allocation to reproduction between the sexes (Lloyd and Webb, 1977; Delph, 1999). However, some studies have reported that vegetative growth in male and female plants did not differ significantly, despite the larger reproductive allocation in females than in males (Sakai and Burris, 1985; Lovett-Doust and Lovett-Doust, 1988; Cipollini and Stiles, 1991). These reports suggest that not all the species examined showed the relative reproductive cost between the sexes.
Several studies have focused on the modularity of woody species, which are hierarchically constructed by reiteration of modules such as leaves, shoots, branches and stems (White, 1979). The modules reflecting the relative reproductive costs differ among species (Cipollini and Whigham, 1994; Obeso, 1997; Bañuelos and Obeso, 2004). For example, reproductive costs were noticeable at the genet, ramet, shoot and leaf levels in Lindera benzoin (Cipollini and Whigham, 1994). Conversely, in Rhamnus alpinus reproductive cost could be detected at the whole-plant level, but not at the branch level (Bañuelos and Obeso, 2004). Hence, the reproductive cost at one module level (i.e. leaf, shoot and branch) may not reflect the cost at the whole-plant level. In addition, the degree of modular integration in resource allocation can vary depending on the timing and stage of reproduction (e.g. Mitchell et al., 2004). These results suggest that reproductive allocation in dioecious plants may be related to the level of the modules and the phenology of reproduction, which delineate the relative reproductive costs between males and females.
Some studies have suggested that female-biased reproductive allocations are compensated through several sex-specific mechanisms, such as the timing of vegetative and reproductive allocation (Delph, 1990; Milla et al., 2006), microhabitat partitioning (Dawson and Ehleringer, 1993), and photosynthesis by fruits (Watson and Casper, 1984). These compensation mechanisms can result in equal reproductive costs in males and females (see Obeso, 2002). Delph (1990) found that differences in the timing of vegetative and reproductive allocation allow female plants of Hebe subalpina, which flowers after a period of vegetative growth, to compensate for the reproductive costs; females allocate more resources to vegetative growth at pre-anthesis, due to smaller reproductive allocation at flowering than males. These results suggest that annual vegetative growth between sexes is equivalent, although the study was only at one modular level. However, cost compensation by more intensive vegetative allocation in females was not detected in other species that flower early in the growing season, e.g. Lindera benzoin (Cipollini and Whigham, 1994) and Ilex aquifolium (Obeso, 1997). The notable point of these studies is that the relative reproductive costs may differ depending on the types of phenophase (i.e. physiological stage of development), because cost compensation through intensive vegetative allocation at pre-anthesis does not overlap with resource-demanding activities such as leafing and reproductive allocation in phenophase-sequencers, but does overlap with resource-demanding activities in the early growing season in phenophase-overlappers.
Rhus javanica and R. trichocarpa (Anacardiaceae) are dioecious, deciduous woody species. Both trees have leaves that open at about the same time in spring, but they differ in flowering phenology. Rhus javanica has large terminal inflorescences and flowers in late August, whereas R. trichocarpa has axillary inflorescences and flowers in June (Kato et al., 1990). These differences in phenology imply that the periods of leafing and developing inflorescences in R. trichocarpa would overlap (i.e. phenophase-overlapper), whereas those in R. javanica would occur in sequence (i.e. phenophase-sequencer). Therefore, on the basis of previous literature, we would expect that: (1) females incur higher reproductive costs than males in R. javanica and R. trichocarpa; and (2) R. javanica, which first produces leaves and then allocates resources to reproduction, has lower reproductive costs than R. trichocarpa, where leafing and reproductive allocation occur simultaneously.
The objectives of this comparative study therefore were: (1) to clarify differences in relative reproductive cost between males and females by comparing reproductive allocation in R. javanica and R. trichocarpa at the shoot and whole-plant level; (2) to compare species-specific reproductive allocation; and (3) to consider the mechanisms that cause differences in relative reproductive costs between sexes and species.
MATERIALS AND METHODS
Study site
The study was conducted at the Asiu Forest Research Station (AFRS) of Kyoto University, in the northern part of Kyoto Prefecture, western Japan (35°18′N, 135°43′ E; elevation 750 m a.s.l.). The mean air temperature and annual precipitation at the closest weather station are 11·9 ºC and 2298·3 mm, respectively (Kyoto University Field Science Education Research Center, 2007). The study area is covered by a cool-temperate conifer–hardwood forest, dominated by the evergreen conifer Cryptomeria japonica and deciduous broad-leaf species such as Fagus crenata, Acer sieboldianum and Quercus crispula (Hirayama and Sakimoto, 2003).
Study species
Rhus trichocarpa and R. javanica (Anacardiaceae) occur naturally throughout the mountains and foothills of south-eastern to eastern Asia, including from Hokkaido to Okinawa in Japan, and in Korea and China (Ohwi and Kitagawa, 1992). Both species are commonly found on disturbed sites, and R. javanica is usually larger than R. trichocarpa.
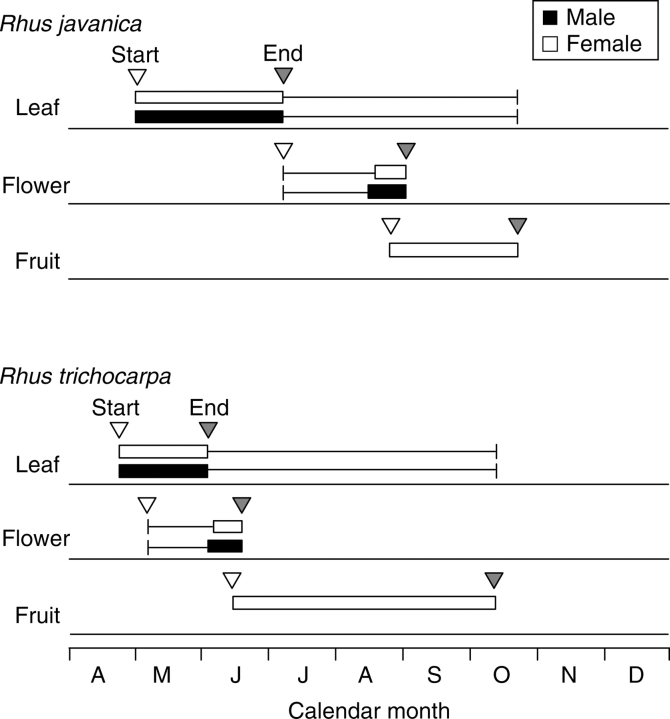
Rhus trichocarpa has axillary inflorescences with numerous flowers, whereas R. javanica has terminal and raceme inflorescences with numerous flowers on current-year shoots (Fig. 1). The sexes of flowers of R. javanica and R. trichocarpa are distinguished by colour: in R. javanica, male pollen-producing flowers are whitish-yellow, whereas female flowers are whitish; in R. trichocarpa, male flowers are yellowish (due to the anthers) whereas female flowers are greenish (S. Matsuyama, pers. obs.). To describe leafing and flowering phenology of R. javanica and R. trichocarpa, six trees (three males, three females) were selected for each species, and five current-year shoots per tree were marked on 1 May, 2004. For the marked shoots, the number of unfolded leaves per shoot was counted once a week. At the same time, the following dates were recorded: (1) appearance of developing inflorescences; (2) flower opening; and (3) fruit development. According to our semi-quantitative survey, leaf production and shoot elongation ceased in June in R. trichocarpa and in July in R. javanica (Fig. 2). In R. javanica, developing inflorescences appear after all leaves have unfolded, and anthesis occurs in late August, whereas R. trichocarpa simultaneously develops inflorescences and leaves in spring, and flowers open as soon as all leaves have unfolded. There were no trees that changed sex during this study. Rhus javanica and R. trichocarpa mainly grows from seedlings, although R. trichocarpa sometimes forms clump (S. Matsuyama, pers. obs.). The seedlings of R. javanica and R. trichocarpa probably originated from parents within the study site, because both species were scattered around the roadside in Research Station.
Fig. 1.
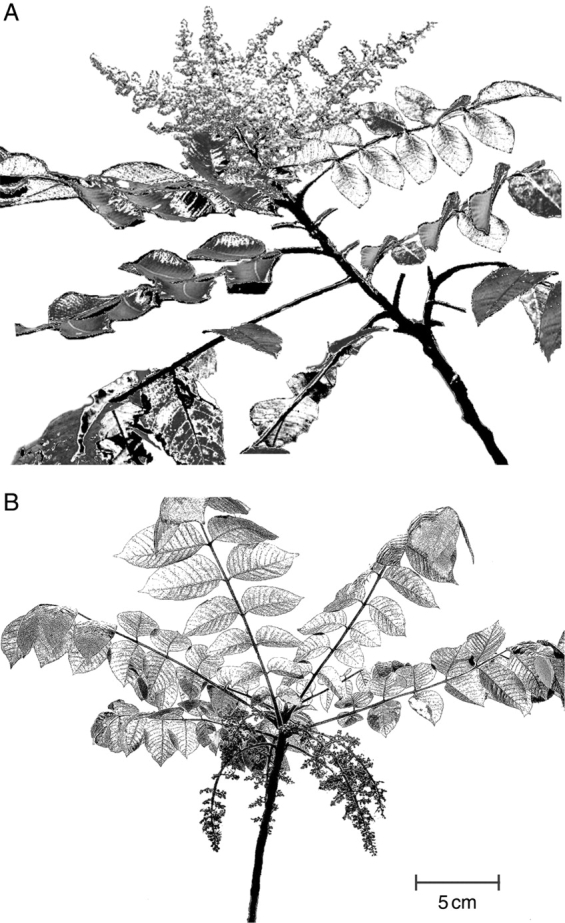
Sketch of a current-year shoot at anthesis: (A) Rhus javanica and (B) R. trichocarpa.
Fig. 2.
Phenology of leafing, flowering and fruiting in Rhus javanica and R. trichocarpa in 2004. Leafing: the box indicates the period from the date when the first leaf unfolded on at least one tree to the date when the number of leaves per shoot was maximum on all three trees; the bar indicates the date on which 80 % of the maximum leaf number was defoliated for all three trees. Flowering: the bar indicates the date on which a developing inflorescence was first observed on at least one tree; the box indicates the period when an open flower was observed on at least one tree. Fruiting: the box indicates the period from when a developing fruit was first observed on at least one tree, to the end of leafing.
Experimental design
In 2001, 40 trees of R. javanica (20 male, 20 female) and 50 of R. trichocarpa (25 male, 25 female) were randomly selected, which were all reproductively mature and in unshaded areas, such as the forest edge at the roadside. The genotypes of the selected trees were not investigated. Twenty trees each (ten males, ten females) of R. javanica and R. trichocarpa were used to examine whole-plant reproductive behaviour and vegetative growth from 2001 to 2004. Ten trees (five males, five females) of each species were used for shoot sampling in order to examine reproductive and vegetative allocation. Shoot sampling was conducted for R. javanica in 2002, and for R. trichocarpa in 2003. For investigation of shoot reproductive costs, ten trees (five males, five females) of R. javanica and 20 trees (ten males, ten females) of R. javanica were used in 2002. The diameter at breast height (DBH), tree height and number of current-year shoots per tree were measured for all trees (Appendix); the ranges of these parameters overlapped between sexes for the two species.
Whole-plant reproductive allocation and vegetative growth
The basal area increment (BAI) of selected trees was measured in order to examine differences in vegetative growth between sexes at the tree level. DBH was measured at fixed point in April (DBHApr) and December (DBHDec) in 2003 and 2004, and BAI was calculated as:
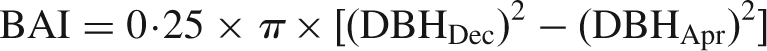 |
The number of current-year shoots per tree and the ratio of reproductive to total current-year shoots per tree was also monitored from 2001 to 2004. A record was made as to whether each current-year shoot produced an inflorescence.
Shoot reproductive allocation, costs and vegetative allocation
Six current-year shoots with inflorescences from trees of R. javanica were randomly harvested in August 2002 (30 male, 30 female), and four current-year shoots with inflorescences from trees of R. trichocarpa (20 male, 20 female) in June 2003, when both species reached anthesis. These samples were divided into shoots, leaves and inflorescences, and the number of leaves and inflorescences per shoot was determined. One inflorescence was selected from each sampled shoot, and the number of flowers per inflorescence was determined. In addition, leaf area per leaf was measured for five leaves from each sampled shoot in order to calculate the specific leaf area (150 male, 150 female in R. javanica; 100 male, 100 female in R. trichocarpa). Twenty flowers per inflorescence from trees of both species were collected and weighed (five males, five females in each case). Using the same females selected in the flowering sampling, current-year shoots with infructescences were harvested in October, when the fruits of both species were ripe: three shoots from female R. javanica (15 infructescences) and four shoots from female R. trichocarpa (20 infructescences). The ranges of the sampled shoot lengths overlapped between the samples at flowering and at fruiting time (see Table 1). One infructescence from the sampled shoot was selected and the number of individual fruits within it was counted. All samples were then dried for 48 h at 80 °C and weighed.
Table 1.
Annual reproductive allocation and related parameters for shoots sampled at flowering and fruiting from female and male trees of Rhus javanica in 2003 and R. trichocarpa in 2004. Values are mean ± s.e.
|
R. javanica |
R. trichocarpa |
|||||
|---|---|---|---|---|---|---|
| Variable | Female (5) | Male (5) | P | Female (5) | Male (5) | P |
| Shoot length at flowering (cm) | 20·50 ± 3·74 (30) | 19·69 ± 3·59 (30) | 0·721 | 9·08 ± 1·19 (20) | 8·71 ± 0·90 (20) | 0·859 |
| Weight of inflorescences per shoot (g) | 2·44 ± 0·24 (30) | 6·44 ± 0·72 (30) | <0·001 | 0·97 ± 0·21 (20) | 2·08 ± 0·23 (20) | 0·039 |
| No. flowers per inflorescence | 2492·0 ± 273·7 (30) | 9683·3 ± 1326·3 (30) | 0·004 | 253·2 ± 32·4 (20) | 280·9 ± 30·3 (20) | <0·001 |
| No. inflorescences per shoot | 1·0 ± 0·0 (30) | 1·0 ± 0·0 (30) | 1·000 | 4·3 ± 0·4 (20) | 6·8 ± 0·3 (20) | <0·001 |
| Dry weight of 20 flowers (mg) | 10·90 ± 1·61 (5) | 9·58 ± 0·99 (5) | 0·505 | 3·42 ± 0·46 (5) | 6·96 ± 0·64 (5) | 0·028 |
| Shoot length at fruiting (cm) | 19·22 ± 1·40 (15) | 7·81 ± 0·49 (20) | ||||
| Weight of infructescences per shoot (g) | 12·51 ± 1·13 (15) | 5·31 ± 0·99 (20) | ||||
| No. fruits per infructescence | 1167·3 ± 129·5 (15) | 72·2 ± 10·2 (20) | ||||
| Annual reproductive allocation per shoot (g) | 12·40 ± 1·39 (5) | 6·44 ± 1·61 (5) | 0·013 | 5·35 ± 1·14 (5) | 2·08 ± 0·35 (5) | 0·043 |
Numbers in parentheses indicate sample size.
Values of P are from LMM for sampled shoot length at flowering; from t-tests for annual reproductive allocation, weight of inflorescences per shoot and dry weight of 20 flowers; and from GLMM-PQL for numbers of flowers per inflorescence, fruits per infructescence and inflorescences per shoot.
The weight of inflorescences per shoot was defined as the annual reproductive allocation per shoot of each male (RAmale). The annual reproductive allocation per shoot of each female tree (RAfemale) was calculated as:
where DWinfru, DWflower, Nflower, Nfruits and Ninflo denote, respectively, the dry weight of infructescences per shoot, the dry weight of a flower, the number of flowers per inflorescence, the number of fruits per infructescence, and the number of inflorescences per shoot. The dry average weight of a flower was calculated from the dry weight of 20 flowers.
The reproductive shoot production and reproductive performance in the following year were investigated for all reproductive 1-year-old shoots in order to evaluate the cost for subsequent reproduction at the shoot level. For the selected trees, all 1-year-old shoots were classified as vegetative or reproductive based on the inflorescence scar. For each 1-year-old shoot, the numbers of reproductive and total current-year shoots per 1-year-old shoot were counted, and the percentage of reproductive current-year shoots per reproductive 1-year-old shoot was calculated (246 male, 271 female in R. javanica; 204 male, 102 female in R. trichocarpa). The effect of sex on reproductive performance in the following year (2002) was investigated at the shoot level, with the type of 1-year-old shoot also being considered (i.e. vegetative or reproductive). Twenty 1-year-old shoots (ten vegetative, ten reproductive) per tree for R. javanica and ten 1-year-old shoots (five vegetative, five reproductive) per tree for R. trichocarpa were randomly selected to make a total of 100 1-year-old shoots (50 vegetative, 50 reproductive) for both sexes of both species, and the status of current-year shoots for each selected 1-year-old shoot was recorded. Both Rhus species form a thin monolayer canopy (S. Matsuyama, pers. obs.), but position within the canopy was not considered for the selected shoots. The status of 1-year-old shoots in the next year was defined as reproductive when the 1-year-old shoots had at least one reproductive current-year shoot, or vegetative when the 1-year-old shoots had no reproductive current-year shoots.
Statistical analysis
All statistical analyses were performed using the software R 2·4·0 (R Development Core Team, 2006). The effect of sex was examined for all analyses in this study. A linear mixed model (LMM; ‘LME’ in the NLME package) was used for the basal area increment, and a generalized linear mixed model (GLMM; ‘GLMM-PQL’ in the R package MASS; Venables and Ripley, 2002) was used for the number of current-year shoots per tree and for the ratio of reproductive to total current-year shoots per tree. The effects of year and interaction between sex and year, and the random effect of tree height were also analysed for the analysis of whole plant reproductive cost. GLMMs were used with a Poisson error and a log-link function for count data, and with a binomial error and a log-link function for ratio data. The LMM and GLMMs were used for multi-comparisons using Bonferroni's correction.
The effect of sex on the annual reproductive allocation per shoot and the dry weight of 20 flowers was analysed using t-tests. The effect of sex analysed by using a LMM (‘LME’ function in the NLME package) for the weight of inflorescences, leaves per shoot, leaf weight per shoot, leaf area per leaf and specific leaf area, and a GLMM (‘GLMM-PQL’ in the R-package MASS; Venables and Ripley, 2002) was used for the number of flowers per inflorescence and the number of leaves per shoot, in order to consider the random factor of variance within an individual. The GLMMs were used with a Poisson error and a log-link function. The effect of sex on the number of current-year shoots and the percentage of reproductive current-year shoots per reproductive 1-year-old shoot were analysed by using a LMM (‘LME’ function in the NLME package) to consider the variance within an individual as a random factor. Data for the number of current-year shoots and the percentage of reproductive current-year shoots per reproductive 1-year-old shoot were log- and arcsine-transformed, respectively.
The effects of sex on the percentage of reproductive shoots in the following year on reproductive and vegetative 1-year-old shoot was analysed by using a GLMM with binomial error and a log-link function, in order to consider the variance within an individual as a random factor. The effect of the type of 1-year-old shoot (i.e. reproductive or vegetative) and the interaction between sex and the type of 1-year-old shoot were also analysed. For R. trichocarpa, the effects of the type and sex of 1-year-old shoots was analysed; however, it was not possible to analyse their interaction because the percentage of reproductive shoots in the following year on a reproductive 1-year-old shoot of females was zero.
RESULTS
Shoot annual reproductive allocation
For individual reproductive shoots, the annual reproductive allocation per shoot of female Rhus javanica was approx. 1·9 times that of males; for female R. trichocarpa it was approx. 2·6 times that of males, because infructescences are much heavier than inflorescences (Table 1). Conversely, the weight of inflorescences per shoot of R. javanica males was approx. 2·6 times that of females, and that of R. trichocarpa males was approx. 2·1 times that of females, because males had more inflorescences per shoot, more flowers per inflorescence and heavier flowers than females (Table 1).
Whole-plant reproductive costs
The effects of year, sex and their interaction with respect to the BAI were not statistically significant in either species (Table 2).
Table 2.
Basal area increments (BAI) for male and female trees of Rhus javanica and R. trichocarpa in 2003 and 2004.
(A) Means and s.e. Numbers in parentheses indicate sample size
|
R. javanica |
R. trichocarpa |
|||
|---|---|---|---|---|
| Variable | Female (10) | Male (10) | Female (10) | Male (10) |
| BAI in 2003 (cm2) | 16·063 ± 2·426 | 13·613 ± 3·548 | 0·905 ± 0·207 | 0·669 ± 0·186 |
| BAI in 2004 (cm2) | 12·846 ± 2·054 | 13·890 ± 3·576 | 0·640 ± 0·130 | 0·651 ± 0·176 |
| (B) t- and P- values from LMM | ||||||
|---|---|---|---|---|---|---|
|
R. javanica |
R. trichocarpa |
|||||
| Variable | d.f. | t | P | d.f. | t | P |
| Sex | 1 | 1·504 | 0·149 | 1 | 0·507 | 0·618 |
| Year | 1 | 0·818 | 0·423 | 1 | 1·198 | 0·246 |
| Sex × Year | 3 | 0·970 | 0·345 | 3 | 1·051 | 0·307 |
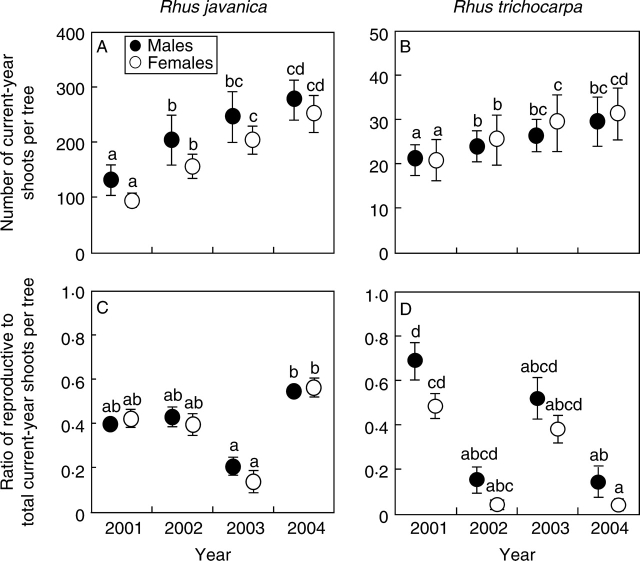
The ratio of reproductive to total current-year shoots per tree was not significantly different between males and females for each year for both R. javanica and R. trichocarpa (GLMM-PQL, sex: R. javanica, d.f. = 7, t = 0·835, P = 0·415; R. trichocarpa, d.f. = 7, t = 1·795, P = 0·089; Fig. 3); however, the ratio of reproductive to total current-year shoots per tree was significantly different among years (GLMM-PQL, year: R. javanica, d.f. = 3, t = 3·102, P = 0·003; R. trichocarpa, d.f. = 3, t = 3·167, P < 0·001). For R. javanica, the ratio of reproductive to total current-year shoots per tree was highest in 2004 and lowest in 2003, whereas the ratio in 2001 was significantly higher than that in 2002 and 2004 in R. trichocarpa (Fig. 3). In terms of the number of current-year shoots per tree, the effects of the interaction between year and sex were not significant for either species, although the number of current-year shoots per tree significantly increased with time (years) for both R. javanica and R. trichocarpa (Fig. 3).
Fig. 3.
(A, B) Number of current-year shoots per tree and (C, D) ratio of reproductive to total current-year shoots per tree for female and male Rhus javanica (A, C) and R. trichocarpa (B, D). Bars indicate s.e. and different letters indicate statistical differences at P = 0·05 from a two-group comparison using a general linear mixed model (GLMM-PQL, with Bonferroni's correction).
Shoot reproductive costs
In R. javanica, the percentage of reproductive current-year shoots was significantly larger on reproductive 1-year-old shoots than on vegetative 1-year-old shoots (Table 3), whereas the effects of sex and the interaction between the type of 1-year-old shoot and sex was not significant. In R. trichocarpa, the percentage of reproductive current-year shoots on 1-year-old shoots for females was significantly smaller than that for males. The percentage of reproductive current-year shoots was significantly smaller on reproductive 1-year-old shoots than on vegetative 1-year-old shoots for R. trichocarpa (Table 3).
Table 3.
Percentages of reproductive shoots in the following year (2002) on reproductive and vegetative 1-year-old shoots, for female and male trees of Rhus javanica and R. trichocarpa
(A) Means and s.e. Numbers in parentheses indicate sample size
|
R. javanica |
R. trichocarpa |
|||
|---|---|---|---|---|
| Type of shoot | Female (5) | Male (5) | Female (10) | Male (10) |
| Reproductive 1-year-old shoot (%) | 80·0 ± 4·5 (50) | 82·0 ± 3·7 (50) | 0·0 ± 0·0 (50) | 8·0 ± 6·3 (50) |
| Vegetative 1-year-old shoot (%) | 18·0 ± 4·9 (50) | 14·0 ± 6·0 (50) | 8·0 ± 6·3 (50) | 18·0 ± 6·6 (50) |
| (B) t- and P-values from GLMM-PQL | ||||||
|---|---|---|---|---|---|---|
|
R. javanica |
R. trichocarpa |
|||||
| Variable | d.f. | t | P | d.f. | t | P |
| Sex | 1 | 0·186 | 0·852 | 1 | 2·051 | 0·040 |
| Type of 1-year-old shoot | 1 | 8·325 | <0·001 | 1 | 2·189 | 0·029 |
| Sex × Type of 1-year-old shoot | 3 | 0·572 | 0·567 | – | – | – |
– Interaction was not included in the analysis in R. trichocarpa, because the percentage of reproductive shoot in the following year on a reproductive 1-year-old shoot of female R. trichocarpa was zero. For details of the experimental design see Materials and Methods.
The effect of sex on the number of current-year shoots per reproductive 1-year-old shoot was not significant in R. javanica and R. trichocarpa (Table 4). For R. javanica, the number of current-year shoots per reproductive 1-year-old shoot was approx. 2·0 for both females and males, compared with approx. 1·1 and 0·9 for females and males, respectively, for R. trichocarpa. The percentage of reproductive current-year shoots per reproductive 1-year-old shoot on females was significantly smaller than that on males in R. trichocarpa, but not in R. javanica (Table 4). In addition, the percentage of reproductive current-year shoots per reproductive 1-year-old shoot in R. javanica was approx. 56·2 % and 61·8 % for females and males, respectively, whereas it was approx. 0 % and 5·6 % for females and males, respectively, in R. trichocarpa.
Table 4.
Number of current-year (2002) shoots and percentage of reproductive current-year shoots per 1-year-old (2001) reproductive shoot for female and male trees of Rhus javanica and R. trichocarpa. Values are mean ± s.e.
|
R. javanica |
R. trichocarpa |
|||||
|---|---|---|---|---|---|---|
| Variable | Female (5) | Male (5) | P | Female (10) | Male (10) | P |
| No. current-year shoots per reproductive 1-year-old shoots | 2·01 ± 0·05 (271) | 2·02 ± 0·05 (246) | 0·920 | 1·08 ± 0·04 (102) | 0·92 ± 0·04 (204) | 0·409 |
| Reproductive current-year shoots per reproductive 1-year-old shoots (%) | 56·24 ± 2·48 (271) | 61·83 ± 2·38 (246) | 0·385 | 0·00 ± 0·00 (102) | 5·55 ± 1·55 (204) | 0·028 |
Numbers in parentheses indicate sample size.
Shoot vegetative allocation
The vegetative allocation to shoots differed between males and females in R. javanica, but not in R. trichocarpa (Table 5). In R. javanica, both the number and weight of leaves per shoot was greater for males than for females, whereas, in R. trichocarpa there were no significant differences between the sexes. There were no significant differences between the sexes for leaf area per leaf and specific leaf area for R. javanica or R. trichocarpa.
Table 5.
Weight and number of leaves per shoot, specific leaf area and area per leaf for shoots sampled at flowering on female and male trees of Rhus javanica and R. trichocarpa. Values are mean ± s.e.
|
R. javanica |
R. trichocarpa |
|||||
|---|---|---|---|---|---|---|
| Variable | Female (5) | Male (5) | P | Female (5) | Male (5) | P |
| Weight of leaves per shoot (g) | 20·72 ± 1·61 (30) | 16·09 ± 1·52 (30) | 0·012 | 12·25 ± 1·22 (20) | 13·96 ± 1·51 (20) | 0·461 |
| No. leaves per shoot | 11·4 ± 0·5 (30) | 9·3 ± 0·5 (30) | 0·013 | 9·1 ± 0·2 (20) | 9·6 ± 0·3 (20) | 0·605 |
| Specific leaf area (cm2 g–1) | 239·3 ± 9·2 (150) | 257·5 ± 11·0 (150) | 0·440 | 173·1 ± 7·7 (100) | 163·3 ± 7·0 (100) | 0·657 |
| Area per leaf (cm2) | 132·9 ± 1·8 (150) | 139·8 ± 2·1 (150) | 0·181 | 113·2 ± 2·4 (100) | 109·5 ± 2·2 (100) | 0·558 |
Numbers in parentheses indicate sample size.
Values of P are from t-tests for the weight of leaves per shoot, from LMM for area per leaf and specific leaf area, and from GLMM-PQL for number of leaves per shoot.
DISCUSSION
Differences in patterns of reproductive allocation, effort and cost
The annual reproductive allocation per shoot of female trees was significantly larger than that of males for both Rhus javanica and R. trichocarpa (Table 2). In addition, the number of current-year shoots and the ratio of reproductive to total current-year shoots per tree was not different between the sexes (Fig. 3), indicating that the number and type of shoots was not drastically different between sexes. These results showed that the annual reproductive allocation of females was larger than that of males at the shoot and whole-plant levels for the two species. Reproductive allocation usually depends on available resources for individuals (e.g. light, microhabitat). For both R. javanica and R. trichocarpa at this open site, the total leaf and inflorescence weight per shoot for female trees was not larger than that for males (Tables 1 and 5), and the number and type of current-year shoots per tree also did not differ between sexes (Fig. 3). These results suggest that available resources at the whole-plant level may not differ between the sexes. Therefore, the larger reproductive allocation in females than in males was caused by differences in resource requirements for reproduction between sexes, not by microhabitat or light conditions.
Differences in the relative reproductive cost between male and female at the whole-plant level were not detected in R. javanica and R. trichocarpa; the ratio of reproductive current-year shoots per tree (Fig. 3) and BAI did not differ between the sexes (Table 2). Furthermore, the reproductive cost at the shoot level was clear in R. trichocarpa, but not in R. javanica (Tables 3 and 4): the reproductive performance and reproductive shoot production in the following year were smaller in females than males in R. trichocarpa, but not in R. javanica (Tables 3 and 4). Annual reproductive allocation negatively correlates with vegetative growth and subsequent reproduction at the whole-plant level in most dioecious plants; the larger annual reproductive allocation in females than in males results in less vegetative growth (Allen and Antos, 1993; Cipollini and Whigham, 1994; Rocheleau and Houle, 2001; Bañuelos and Obeso, 2004), less subsequent growth (Obeso, 1997; Bañuelos and Obeso, 2004) and less subsequent reproduction (Ågren, 1988; Popp and Reinartz, 1988; Oyama, 1990; Cipollini and Stiles, 1991; Nicotra, 1999) in females than in males. Furthermore, several studies have shown that reproductive costs might be detected at both whole-plant and lower-module levels (Cipollini and Whigham, 1994; Obeso, 1997). Therefore, the annual reproductive allocation in R. javanica and R. trichocarpa in this study suggest that larger reproductive costs of females should be observed in vegetative growth and reproduction at the shoot and/or whole-plant level for both species. However, the results indicated that the relative reproductive costs at the whole-plant level in R. javanica and R. trichocarpa differ from those at the shoot level. Bañuelos and Obeso (2004) found less growth in females than in males in Rhamnus alpinus at the individual level, but not at the shoot or the branch level, and suggested that the degree of the trade-off between reproduction and other functions was different at the different module levels, because the whole plant may consisted of subunits that are physiologically different (Watson and Casper, 1984; Sprugel et al., 1991; Obeso et al., 1998). Therefore, the results of our study may reflect the fact that the trade-off between current and subsequent reproduction at shoot level was different from that at the whole-plant level in R. trichocarpa, suggesting that the relative reproductive costs at lower-module levels might not only enhance but may also diminish the whole-plant costs through the integration of modules in woody species.
In R. trichocarpa, the relative reproductive costs were detected at the shoot level but not at the whole-plant level (Tables 2–4, Fig. 3). Cipollini and Stiles (1991) reported equivalent vegetative growth between the sexes and lower flowering frequency of females than males in Nyssa sylvatica. These authors suggested that, in their whole-plant study, the trade-off between current and subsequent reproduction counterbalanced the trade-off between reproduction and vegetative growth. Therefore, the reduced future reproductive allocation at the shoot level might counterbalance the relative reproductive cost of the other functions at the shoot and the whole-plant level; thus the cost between the sexes at the whole-plant level might not have been apparent in R. trichocarpa.
On the other hand, relative reproductive costs were not detected at either the whole-plant or shoot level in R. javanica. Several dioecious plants show no difference between sexes in reproductive costs, but they have compensative mechanisms, such as larger resource allocation to leaves and drastically different flowering phenology of sexes (Delph, 1999). Thus, no obvious relative reproductive cost in R. javanica might result from a compensative mechanism. In R. javanica, the number and weight of leaves per shoot were significantly higher in females than in males, but this trend was not observed for R. trichocarpa (Table 5), indicating that both vegetative and reproductive allocation per shoot are larger in females than males in R. javanica but not in R. trichocarpa. Higher vegetative and reproductive allocation in females than males has been reported in a number of studies (Popp and Reinartz, 1988; Korpelainen, 1992; Bram and Quinn, 2000). Popp and Reinartz (1988) showed that females of Xanthoxylum americanum allocated more biomass to the growth of leaves on flowering shoots and to reproductive tissue than males, and proposed that a greater investment in leaf tissue for flowering and fruiting may be required to support the additional energy drain of developing fruits. Thus, females of R. javanica might compensate for the investment in reproduction by developing additional leaves on reproductive current-year shoots. Furthermore, the results for number and percentage of current-year shoots per reproductive 1-year-old shoot showed that the average 1-year-old shoot produces one vegetative and one reproductive current-year shoot in R. javanica (Table 4). Sprugel et al. (1991) found that when local sources of photosynthate are eliminated (e.g. by shoot defoliation), there is an influx of photosynthate from other plant parts. Obeso (1998) demonstrated that in Ilex aquifolium, fruit production on completely defoliated branches did not differ from that on untreated branches, suggesting that neighbouring shoots contribute to fruit maturation when local photosynthate production is reduced. Hence the number and type of current-year shoots per reproductive 1-year-old shoot suggest that the neighbouring shoots might also help to compensate for reproductive costs in R. javanica. However, in this species, the additional leaves on reproductive shoots may be more important for cost compensation in females than resource transport from neighbouring shoots, because there were no obvious differences between sexes in the number and type of neighbouring shoots (Table 4).
Differences between species in phenology patterns
Reproductive costs at the shoot level were detected in R. trichocarpa but not in R. javanica, whereas larger vegetative allocation to shoots was detected in R. javanica but not in R. trichocarpa. These results suggest that vegetative allocation at the shoot level may be related to differences in the costs at the shoot level between the species. Typically, the amount of vegetative and reproductive allocation depends on resource availability: e.g. habitat (Popp and Reinartz, 1988) and phenology pattern (Shitaka and Hirose, 1998). Therefore, in our comparative study of two Rhus species at an open site, the difference between species in resource use might reflect, at least, differences in the phenophase between species (i.e. physiological stage of development).
The leaf phenological patterns of R. javanica and R. trichocarpa do not differ between the species, whereas the flowering and fruiting phenology patterns do: R. javanica is a phenophase-sequencer, with leafing followed by flowering and fruiting, whereas R. trichocarpa is a phenophase-overlapper, simultaneously producing leaves and flowers, followed by fruiting (Fig. 2). Rhus javanica and R. trichocarpa also have differences in shoot architecture; R. javanica forms terminal inflorescences whereas R. trichocarpa has axillary inflorescences on current-year shoots (Fig. 1). Kudo (2006) showed that abiotic and biotic factors (i.e. pollination, herbivory, seed dispersal and germination) relate to the evolution of flowering phenologies. In a conceptual model study, Diggle (1999) suggested that shoot architecture enhances or constrains the evolution of flowering phenology through vegetative development patterns, such as leaf production pattern. Therefore, the differences in flowering and fruiting phenologies between R. javanica and R. trichocarpa may have developed through different shoot architectures. However, several studies have shown that allocation to fruits is the single largest component of resource allocation (e.g. Allen and Antos, 1988; Antos and Allen, 1994) and therefore the timing of fruit development can be an important factor when considering overall allocation of reproductive resources. Therefore the difference in relative reproductive cost between a phenophase-overlapper and a phenophase-sequencer might reflect the different timing of allocation of resources to flowering and fruiting during an annual growing season.
Females of several sequencers show larger vegetative and reproductive allocation than do males (Conn and Blum, 1981; Delph, 1990; Korpelainen, 1992; Bram and Quinn, 2000). Delph (1990) found that female Hebe subalpina, which has vegetative allocation before flowering, allocates more resources to vegetative tissues than males do in the early growing season, resulting in the compensation of larger reproductive allocation for females. Larger vegetative and reproductive allocation in females has also been reported in dioecious annual herbs such as Rumex acetosa (Korpelainen, 1992) and Amaranthus cannabinus (Bram and Quinn, 2000). In general, annual herbs form flowers following periods of vegetative growth, i.e. they are phenophase-sequencers. Such studies suggest that the compensation mechanism of additional vegetative allocation for females is not specific to R. javanica, and also suggest that the compensation mechanism is related to the sequencer's phenology pattern. In contrast, for phenophase-overlappers, vegetative and reproductive allocations early in the growing season are a trade-off for available resources. In Styrax obassia, which flowers in early summer, Miyazaki et al. (2002) found that leaf mass per area and leaf nitrogen concentration were lower in reproductive than in non-reproductive shoots, but the number of leaves did not differ between shoot types, thus indicating a trade-off between vegetative and reproductive allocation early in the growing season in phenophase-overlappers. The trade-off in the early growing season may prevent compensation through additional leaf production in phenophase-overlappers because (1) additional vegetative allocation decreases reproductive allocation to flowering, and (2) the diminished reproductive allocation at flowering may also decrease the attractiveness to pollinators and hence the chance for mating. This study showed that the type of phenophase might be related to differences in leaf production between sexes in the two Rhus species. The results also suggest that the type of phenophase (e.g. leafing and flowering phenology) affects reproductive costs at the shoot and whole-plant levels through the development of compensation mechanisms.
ACKNOWLEDGEMENTS
We owe special thanks to Dr K. Hirayama for her invaluable help and advice in the field studies, laboratory analysis and writing of this paper, to Mr K. Morishita for comments and field assistance, and to Dr N. Osawa for his invaluable help and advice in writing this paper. We deeply appreciate comments and suggestions on this study by Prof. S. Shibata and other members of the Laboratory of Silviculture. Finally, we thank the staff of Asiu Forest Research Station for permission and support for our field study.
APPENDIX
Ranges of height, DBH and number of current-year shoots per tree for selected female and male trees of Rhus javanica and R. trichocarpa in 2001. Sample sizes are given in brackets.
(A) For monitoring of the basal area increment, the number of current-year shoots and the ratio of reproductive to total current-year shoots per whole plant
|
R. javanica |
R. trichocarpa |
|||
|---|---|---|---|---|
| Variable | Female (10) | Male (10) | Female (10) | Male (10) |
| Height (m) | 5·7–9·4 | 5·5–10·5 | 2·1–5·2 | 2·4–4·9 |
| DBH (cm) | 7·8–13·6 | 8·5–20·0 | 1·2–7·1 | 1·7–7·1 |
| No. of current-year shoots per tree | 33–157 | 20–286 | 6–49 | 5–59 |
(B) For shoot harvesting
|
R. javanica |
R. trichocarpa |
|||
|---|---|---|---|---|
| Variable | Female (5) | Male (5) | Female (5) | Male (5) |
| Height (m) | 5·9–8·0 | 5·3–8·7 | 3·5–5·1 | 2·4–4·5 |
| DBH (cm) | 5·4–15·7 | 8·3–15·2 | 3·0–6·3 | 2·5–5·0 |
| No. of current-year shoots per tree | 58–245 | 58–239 | 13–31 | 8–49 |
(C) For counting the number of reproductive and total current-year shoots per 1-year-old shoot
| R. javanica | R. trichocarpa | |||
|---|---|---|---|---|
| Variable | Female (5) | Male (5) | Female (10) | Male (10) |
| Height (m) | 5·1–9·4 | 5·5–9·0 | 3·6–5·2 | 3·0–4·7 |
| DBH (cm) | 10·6–14·0 | 8·4–15·2 | 2·8–7·1 | 2·4–7·1 |
| No. of current-year shoots per tree | 89–237 | 91–233 | 11–49 | 12–59 |
LITERATURE CITED
- Ågren J. Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamaemorus. Ecology. 1988;69:962–973. [Google Scholar]
- Allen GA, Antos JA. Relative reproductive effort in males and females of the dioecious shrub Oemleria cerasiformis. Oecologia. 1988;76:111–118. doi: 10.1007/BF00379608. [DOI] [PubMed] [Google Scholar]
- Allen GA, Antos JA. Sex-ratio variation in the dioecious shrub Oemleria cerasiformis. American Naturalist. 1993;141:537–553. doi: 10.1086/285490. [DOI] [PubMed] [Google Scholar]
- Antos JA, Allen GA. Biomass allocation among reproductive structures in the dioecious shrub Oemleria cerasiformis –a functional interpretation. Journal of Ecology. 1994;82:21–29. [Google Scholar]
- Bañuelos MJ, Obeso JR. Resource allocation in the dioecious shrub Rhamnus alpinus: the hidden costs of reproduction. Evolutionary Ecology Research. 2004;6:397–413. [Google Scholar]
- Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF. Allocating resources to reproduction and defense. BioScience. 1987;37:58–67. [Google Scholar]
- Bram MR, Quinn JA. Sex expression, sex-specific traits, and the effects of salinity on growth and reproduction of Amaranthus cannabinus (Amaranthaceae), a dioecious annual. American Journal of Botany. 2000;87:1609–1618. [PubMed] [Google Scholar]
- Cipollini ML, Stiles EW. Costs of reproduction in Nyssa sylvatica—sexual dimorphism in reproductive frequency and nutrient flux. Oecologia. 1991;86:585–593. doi: 10.1007/BF00318326. [DOI] [PubMed] [Google Scholar]
- Cipollini ML, Whigham DF. Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae) American Journal of Botany. 1994;81:65–75. [Google Scholar]
- Conn JS, Blum U. Differentiation between the sexes of Rumex hastatulus in net energy allocation, flowering and height. Bulletin of the Torrey Botanical Club. 1981;108:446–455. [Google Scholar]
- Dawson TE, Ehleringer JR. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder. Acer negundo. Ecology. 1993;74:798–815. [Google Scholar]
- Delph LF. Sex-differential resource-allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990;71:1342–1351. [Google Scholar]
- Delph LF. Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. New York: Springer-Verlag; 1999. pp. 149–173. [Google Scholar]
- Diggle PK. Heteroblasty and the evolution of flowering phenologies. International Journal of Plant Science. 1999;160:S123–S134. doi: 10.1086/314217. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Sakimoto M. Regeneration of Cryptomeria japonica on a sloping topography in a cool-temperate mixed forest in the snowy region of Japan. Canadian Journal of Forest Research. 2003;33:543–551. [Google Scholar]
- Hoffmann AJ, Alliende MC. Interactions in the patterns of vegetative growth and reproduction in woody dioecious plants. Oecologia. 1984;61:109–114. doi: 10.1007/BF00379095. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Cohen D. Optimal growth schedule of a perennial plant. American Naturalist. 1989;133:480–505. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. Evolutionary ecology of plant reproductive strategies. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Kato M, Kakutani T, Inoue T, Itino T. Insect–flower relationship in the primary beech forest of Ashu, Kyoto: an overview of the flowering phenology and the seasonal pattern of insect visits. Contributions from the Biological Laboratory, Kyoto University. 1990;27:309–375. [Google Scholar]
- Klinkhamer PGL, Kubo T, Iwasa Y. Herbivores and the evolution of the semelparous perennial life-history of plants. Journal of Evolutionary Biology. 1997;10:529–550. [Google Scholar]
- Korpelainen H. Patterns of resource-allocation in male and female plants of Rumex acetosa and R. acetosella. Oecologia. 1992;89:133–139. doi: 10.1007/BF00319025. [DOI] [PubMed] [Google Scholar]
- Kudo G. Flowering phenologies of animal-pollinated plants: reproductive strategies and agents of selection. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 139–158. [Google Scholar]
- Kyoto University Field Science Education Research Center. The 14th Meteorological observations in the Kyoto University Forests. Kyoto: Field Science Education Research Center of Kyoto University; 2007. (in Japanese) [Google Scholar]
- Lloyd DG, Webb CJ. Secondary sex characters in plants. Botanical Review. 1977;43:177–216. [Google Scholar]
- Lovett-Doust J, Lovett-Doust L. Modules of production and reproduction in a dioecious clonal shrub, Rhus typhina. Ecology. 1988;69:741–750. [Google Scholar]
- Milla R, Castro-Diez P, Maestro-Martinez M, Montserrat-Marti G. Costs of reproduction as related to the timing of phenological phases in the dioecious shrub Pistacia lentiscus L. Plant Biology. 2006;8:103–111. doi: 10.1055/s-2005-872890. [DOI] [PubMed] [Google Scholar]
- Mitchell MGE, Antos JA, Allen GA. Modules of reproduction in females of the dioecious shrub Oemleria cerasiformis. Canadian Journal of Botany. 2004;82:393–400. [Google Scholar]
- Miyazaki Y, Hiura T, Kato E, Funada R. Allocation of resources to reproduction in Styrax obassia in a masting year. Annals of Botany. 2002;89:767–772. doi: 10.1093/aob/mcf107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB. Reproductive allocation and the long-term costs of reproduction in Siparuna grandiflora, a dioecious neotropical shrub. Journal of Ecology. 1999;87:138–149. [Google Scholar]
- Obeso JR. Costs of reproduction in Ilex aquifolium: effects at tree, branch and leaf levels. Journal of Ecology. 1997;85:159–166. [Google Scholar]
- Obeso JR. Effects of defoliation and girdling on fruit production in Ilex aquifolium. Functional Ecology. 1998;12:486–491. [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Obeso JR, Alvarez-Santullano M, Retuerto R. Sex ratio, size distributions, and sexual dimorphism in the dioecious tree Ilex aquifolium (Aquifoliaceae) American Journal of Botany. 1998;85:1602–1608. [PubMed] [Google Scholar]
- Ohwi J, Kitagawa M. New flora of Japan. Tokyo: Shibundo (in Japanese); 1992. [Google Scholar]
- Oyama K. Variation in growth and reproduction in the neotropical dioecious palm Chamaedorea tepejilote. Journal of Ecology. 1990;78:648–663. [Google Scholar]
- Popp JW, Reinartz JA. Sexual dimorphism in biomass allocation and clonal growth of Xanthoxylum americanum. American Journal of Botany. 1988;75:1732–1741. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Rocheleau AF, Houle G. Different cost of reproduction for the males and females of the rare dioecious shrub Corema conradii (Empetraceae) American Journal of Botany. 2001;88:659–666. [PubMed] [Google Scholar]
- Sakai AK, Burris TA. Growth in male and female aspen clones – a 25-year longitudinal study. Ecology. 1985;66:1921–1927. [Google Scholar]
- Shitaka Y, Hirose T. Effects of shift in flowering time on the reproductive output of Xanthium canadense in a seasonal environment. Oecologia. 1998;114:361–367. doi: 10.1007/PL00008818. [DOI] [PubMed] [Google Scholar]
- Sprugel DG, Hinckley TM, Schaap W. The theory and practice of branch autonomy. Annual Review of Ecology and Systematics. 1991;22:309–334. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th edn. Berlin: Springer; 2002. [Google Scholar]
- Watson MA, Casper BB. Morphogenetic constraints on patterns of carbon distribution in plants. Annual Review of Ecology and Systematics. 1984;15:233–258. [Google Scholar]
- White J. The plant as a metapopulation. Annual Review of Ecology and Systematics. 1979;10:109–145. [Google Scholar]
- Willson MF. Plant reproductive ecology. New York: John Wiley and Sons; 1983. [Google Scholar]