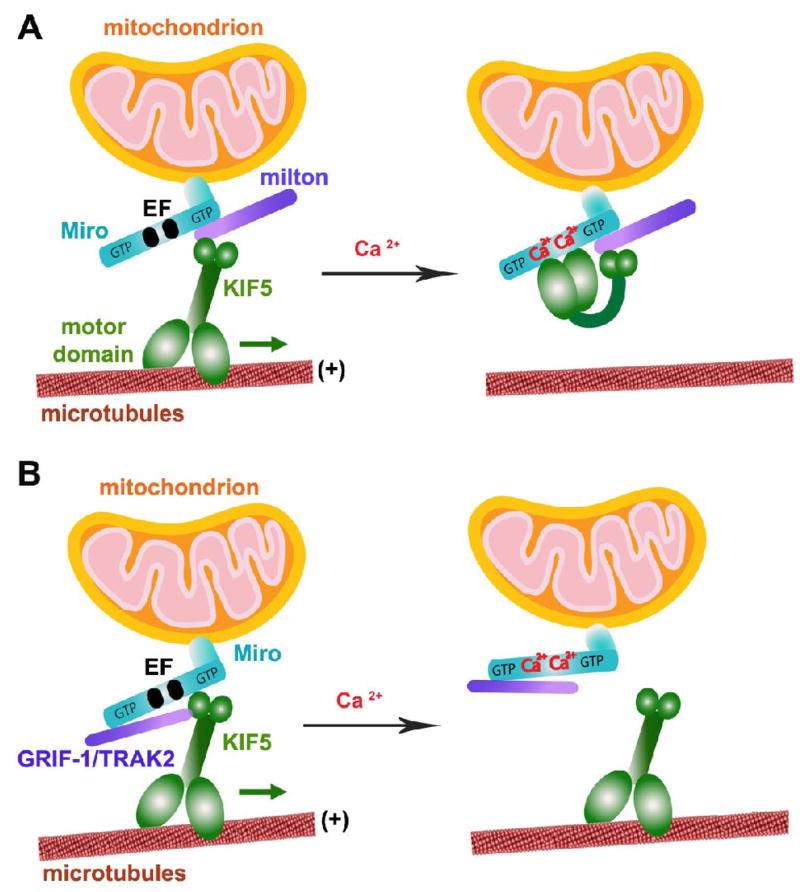
Fig. 3.
Schematic diagram for two proposed models of Miro as a Ca2+ sensor in regulating mitochondrial mobility. Miro contains two GTPase domains and calcium-binding EF hand motifs, thus regulates axonal mitochondrial motility by either GTP hydrolysis or calcium binding in response to calcium signals and synaptic activity. (A) Model 1: Ca2+-binding turns “off” KIF5 engagement with MTs. The tail of KIF5 is linked to Miro via milton in a Ca2+-independent manner, thus leaving its motor domain to engage with MTs. Ca2+-binding to the EF hands triggers the direct interaction of the motor domain with Miro, thus preventing the motor from engaging with MTs (Wang and Schwarz, 2009). (B) Model 2: Ca2+-binding detaches KIF5 from mitochondria. Mitochondrial transport is mediated by linking Miro to KIF5. Ca2+-binding to the EF hands dissociates Miro from KIF5 while KIF5-binding protein GRIF-1/TRAK2 (a mammalian homologue of milton) remains bound to Miro1 (MacAskill et al., 2008; 2009). (The model diagram is adapted with permission from Qian Cai and Zu-Hang Sheng. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron 61, 493–496. 2009).