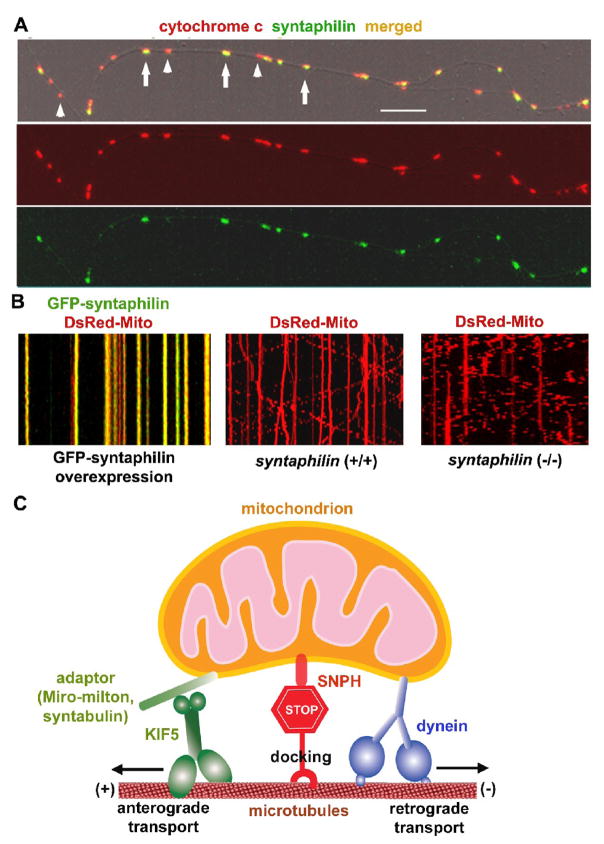
Fig. 4.
SNPH acts as a receptor for docking/anchoring mitochondria at microtubules in axons. (A) Axonal mitochondrial targeting of SNPH in cultured hippocampal neurons. Hippocampal neurons at DIV14 were co-immunostained for SNPH and mitochondrial marker cytochrome c. Arrows point to SNPH-associated mitochondria and arrowheads indicate SNPH-negative mitochondria within an axonal process. Scale bars, 10 μm. (B) SNPH immobilizes axonal mitochondria and deletion of snph gene in mice robustly increases mobility of axonal mitochondria. Mobility of axonal mitochondria was observed in live neurons one week after transfection. Motion data are presented in kymograph, in which vertical lines represent stationary mitochondria and slant lines or curves indicate motile ones. Left panel: wild-type neurons co-transfected at DIV6 with DsRed-mito (red) and GFP-SNPH (green); middle panel: wild-type neurons transfected with DsRed-Mito alone; right panel: snaph (−/−) neurons transfected with DsRed-Mito alone. (C) Schematic diagram for the proposed role of SNPH. SNPH acts as a receptor for docking/anchoring mitochondria in axons and is required for maintaining a large number of axonal mitochondria in a stationary state by interacting with the microtubule-based cytoskeleton. Such a mechanism may enable neurons to maintain proper densities of stationary mitochondria within axons and in the proximity of synapses. (Imaging in Fig. 4A is adapted with permission from Jian-Sheng Kang, Jin-Hua Tian, Philip Zald, Ping-Yue Pan, Cuiling Li, Chuxia Deng, and Zu-Hang Sheng. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148, 2008).