Table 1.
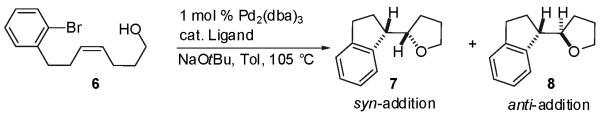 | |||
|---|---|---|---|
| entry | ligand | dr(b) | Isolated Yield (7 + 8) |
| 1 | P[(p-MeO)C6H4]3 | 8:1 | 54% |
| 2 | PPh3 | 5:1 | 27% |
| 3 | P(o-tol)3 | 2:1 | 35% |
| 4 | PCy3 | 2:1 | 48% |
| 5 | DPE-Phos | 1:1 | 49% |
| 6 | DPPE | 1:2 | 46% |
| 7 | DPP-Benzene | 1:2 | 44% |
| 8 | (±)-BINAP | 1:18 | 60% |
Conditions: 1.0 equiv 6, 2.0 equiv NaOtBu, 1 mol % Pd2(dba)3, 4 mol % ligand (monophosphines) or 2 mol % ligand (bis-phosphines), toluene (0.1 M), 105 °C, 3-8 h.
Diastereoselectivities were determined by GC and/or 1H NMR analysis of crude reaction mixtures.
