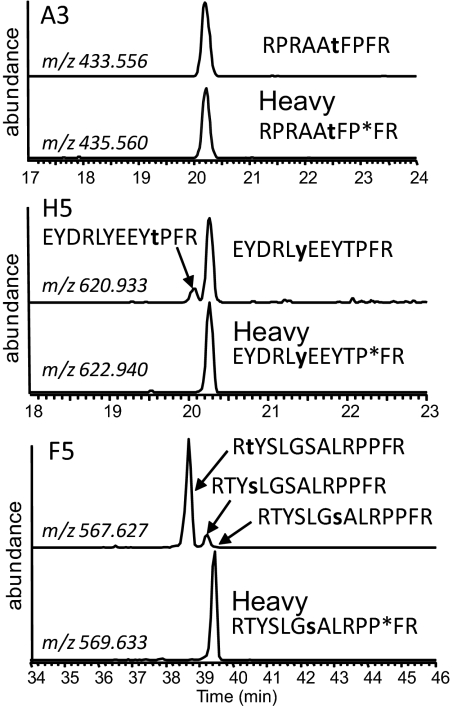
Fig. 2.
Site-specific measurement of peptide phosphorylation rates. Peptides phosphorylated at different Ser/Thr/Tyr residues were resolved by LC and phosphorylation sites were localized by concurrent tandem MS analysis (see Fig. S3 for an example). Perfect co-elution of the internal standard and product facilitated the determination of a site-specific phosphorylation rate. Extracted ion chromatograms were produced using a ± 10 ppm tolerance surrounding the predicted mass-to-charge (m/z) ratio for each phosphopeptide and each internal standard (contains heavy proline residue, indicated by asterisk). Confirmed phosphorylation sites are bold and lowercase. Peptide A3 has a single acceptor site residue, and its phosphorylation product perfectly co-eluted with the internal standard. In contrast, the phosphorylation products of H5 and F5 both contained multiple position isomers. In the case of F5, no site-specific phosphorylation toward the site in the IStd was detected.