Abstract
Patients with chronic myelogenous leukemia have a t(9;22)(q34;q11.2) or variant translocation that results in a BCR-ABL fusion gene. BCR-ABL detection by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) is the standard practice for monitoring residual disease in patients with chronic myelogenous leukemia who receive tyrosine kinase inhibitor therapies. In this study, we describe a patient who tested positive for the BCR-ABL translocation by fluorescence in situ hybridization and cytogenetic analysis but tested negative by qRT-PCR molecular analysis at the time of diagnosis. Further PCR analysis and DNA sequencing with alternative primer sets demonstrated the presence of an e14a3 (also known as b3a3) BCR-ABL fusion. The e14a3 fusion is rare, but may be underreported as a result of many commercially available and laboratory-developed primer sets that fail to detect breakpoints in the ABL gene that are downstream of intron 1. For this patient, if the qRT-PCR assay had been used to monitor disease response/progression after treatment and not in conjunction with fluorescence in situ hybridization or cytogenetics at the time of diagnosis, the negative result would have been misinterpreted as molecular remission.
Chronic myelogenous leukemia (CML) is the first human cancer causally linked to a specific chromosomal abnormality, the Philadelphia chromosome (Ph), which is a product of a reciprocal translocation between chromosome 9 and 22 [t(9;22)(q34;q11.2)]. This particular translocation results in the BCR-ABL gene fusion that was subsequently shown to have transforming activity due to the deregulated tyrosine kinase activity of ABL.1 In CML, >95% of the breakpoints involve the M-bcr region consisting of BCR introns downstream of either exon 13 (e13, previously known as b2) or 14 (e14, previously known as b3) and introns upstream of ABL exon 2 (a2). These BCR-ABL e13a2 and e14a2 fusions result in a 210-kd fusion protein.2 There are two less common breakpoints in the intronic region between the alternative BCR exon 2 known as m-bcr, and between BCR exons 19 and 20, known as μ-bcr, which encode a 190-kd (e1a2) and 230-kd fusion protein (e19a2), respectively.3,4 Rare atypical breakpoints have also been sporadically reported and can be grouped into four categories: BCR breakpoints originating within introns that lie outside M-bcr, m-bcr, or μ-bcr fused to ABL a2; BCR breakpoints occurring within exons fused to ABL a2; typical BCR breakpoints (M-bcr, m-bcr, or μ-bcr) fused to ABL breakpoints located downstream of a2; and transcripts containing intervening sequences between BCR and ABL a2.5
There are multiple methods for detecting the BCR-ABL translocation including cytogenetics, Southern blot, fluorescence in situ hybridization (FISH), and reverse transcription polymerase chain reaction (RT-PCR) (including quantitative qRT-PCR). Each method has advantages and disadvantages. Cytogenetics, Southern blot, and some FISH-based assays should be able to detect essentially all BCR-ABL translocations regardless of the breakpoints, while RT-PCR assays are limited in the breakpoints detected based on the location of the primers and probes. However, cytogenetics, Southern blot, and FISH all have limits of detection of approximately 1–5%, while qRT-PCR assays can detect down to 0.01% or fewer malignant cells. Thus qRT-PCR is the standard test for minimal residual disease monitoring of patients receiving tyrosine kinase inhibitor therapy according to the 2009 National Comprehensive Cancer Network clinical practice guidelines for CML.6
Here we report a case of CML with an e14a3 fusion transcript that showed a positive BCR-ABL FISH result but had a negative result by qRT-PCR. We review the frequency and known biology of this rare fusion and also review the ability of commercially available BCR-ABL qRT-PCR assays to detect this fusion product.
Materials and Methods
Metaphase Cytogenetics and Fluorescence in Situ Hybridization
Bone marrow cells were cultured according to standard cytogenetic laboratory protocols. Twenty G-banded metaphases were evaluated and two karyotypes prepared. FISH was performed following the manufacturer's recommended protocol. LSI BCR/ABL dual color, dual fusion translocation probe set (Abbott Molecular Inc., Des Plaines, IL) was used to identify BCR-ABL fusion genes. This probe set hybridizes to chromosome 22q11.2 (BCR-SpectrumGreen) and to chromosome 9q34 (ABL-SpectrumOrange). A normal cell should show two separate sets of orange and green signals (2O2G), while a cell containing reciprocal t(9;22) shows individual orange and green signals from the normal 9 and 22 chromosomes and two orange/green fusion signals from the derivative 9 and 22 chromosomes (1O1G2F pattern). Positive and negative controls plus 200 interphase cells from the patient were analyzed by two independent scorers.
RNA Isolation and RT-PCR for BCR-ABL Transcript Detection
Total RNA was isolated from patient samples (peripheral blood and bone marrow) using the QIAamp RNA Mini blood kit (Qiagen Inc., Valencia, CA). Isolated RNA was quantified by spectrophotometric analysis at 260 nm and 280 nm.
Routine BCR-ABL transcript detection was performed using the BCR-ABL Mbcr/ABL FusionQuant kit (Ipsogen, New Haven, CT), which amplifies BCR-ABL and ABL in two independent reactions. The one-step RT-PCR reactions consisted of 300 ng of RNA, primer/probe mix (Ipsogen), QuantiTect Probe RT-PCR Master Mix, and QuantiTect RT Mix (Qiagen Inc.). Patient samples, BCR-ABL standards (3), ABL standards (3), and RNA controls (3) were all run in duplicate reactions. Reactions were thermal cycled using the 7900 TaqMan (Applied Biosystems, Foster City, CA) as follows: 50°C for 30 minutes, 95°C for 15 minutes, followed by 50 cycles of 94°C for 15 seconds and 60°C for 1 minute. At completion of run, the data were analyzed and results were reported as the number of BCR-ABL transcripts per 1000 ABL transcripts.
cDNA synthesis was performed using 1 μg of RNA isolated from the bone marrow specimen and the SuperScript III First Strand synthesis kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. PCR reactions contained 1X PCR buffer (Applied Biosystems), 25 nmol dNTPs (Applied Biosystems), 250 pmol forward primer (e1 or e12), and 250 pmol a3 reverse primer (Operon Biotechnologies Inc., Huntsville, AL), 1.5 units of AmpliTaq Gold polymerase (Applied Biosystems), and 5 μl of cDNA in a 50-μl reaction volume. Primer sequences8 were e1 forward primer (5′-TGGAGGAGGTGGGCATCTAC-3′), e12 forward primer (5′-GCAGAGTGGAGGGAGAACAT-3′), and a3 reverse primer (5′-GCTTCACACCATTCCCCATT-3′). PCR amplification was performed using the ABI9700 according to the following: 94°C for 2 minutes, three cycles of 94°C for 30 seconds, 64°C for 30 seconds, and 70°C for 30 seconds, followed by three cycles with annealing temperature decreased to 61°C, another three cycles with annealing temperature decreased to 58°C, then 35 cycles of 94°C for 30 seconds, 57°C for 30 seconds, and 70°C for 30 seconds, and final extension at 70°C for 5 minutes. PCR products were separated by 1% agarose gel electrophoresis and visualized under ultraviolet light after staining with ethidium bromide.
Direct Sequencing
PCR products were purified using QiaQuick reagents (Qiagen) and were cycle sequenced using Big Dye v3.1 reagents (Applied Biosystems) and the forward and reverse PCR primers according to the manufacturer's protocol. Sequencing products were purified with CleanSEQ sequencing purification system (Agencourt Bioscience Corp., Beverly, MA) and automated sequencing performed by capillary electrophoresis on an ABI3700 (Applied Biosystems). Sequences were aligned and examined using Sequencher software (Gene Codes Corp., Inc.).
Case Report
The 81-year-old patient was originally referred for consultation due to a persistently elevated white blood cell count (WBC). The patient reported that he had been followed closely over the past several years due to numerous medical problems including chronic obstructive pulmonary disease, hypertension, congestive heart failure, chronic atrial fibrillation, and insulin-resistant diabetes. Routine blood testing discovered an elevated WBC of 28,000/mm3 without evidence of anemia or thrombocytopenia. His peripheral blood differential was left shifted but showed no peripheral blasts. His red cell indices were unremarkable. His counts were repeated and showed a similar elevation in his WBC with notable left shift. He was referred to a hematologist who pursued a work up for suspected myeloproliferative disease, specifically directed toward a diagnosis of CML. An initial peripheral blood smear was reviewed and found without evidence of acute leukemia or dysplasia. Blood was sent to evaluate for evidence of the Philadelphia chromosome (Ph) by FISH and for the BCR-ABL gene rearrangement by qRT-PCR and both tests were reported as negative. At that time, the patient underwent a bone marrow aspirate and biopsy, which remained consistent with a myeloproliferative disorder showing a hypercellular marrow with a marked myeloid predominance. There was no evidence of increased blasts in the marrow, however the myeloid series was left shifted. The outside hematological pathology report once again raised the question of CML, but noted the limits of their ability to confirm the diagnosis without chromosome or other evidence of the Ph chromosome. At this point, the patient was referred to the Johns Hopkins Sidney Kimmel Cancer Center for a second opinion regarding the diagnosis of a Ph chromosome negative myeloproliferative disorder.
Results and Discussion
After a complete history, physical, and review of the patient's outside records and report, the diagnosis remained unclear, yet concerning for CML. A repeat bone marrow aspiration and biopsy with complete morphological, cytogenetic, and molecular testing was ordered. Bone marrow biopsy showed a markedly hypercellular marrow with myeloid predominance and left shifted granulopoiesis showing complete maturation. Karyotype analysis revealed 46, XY, t(9;22)(q34;q11.2) [20/20] (Figure 1A). FISH confirmed the presence of the BCR-ABL translocation with 92% of cells reporting positive for the translocation with the typical pattern of 1O1G2F (Figure 1B). A peripheral blood specimen with a white blood cell count of approximately 40,000/mm3 also demonstrated 90% BCR-ABL FISH positivity. It is unclear why the outside BCR-ABL FISH results were reported as negative, and we are unable to investigate this any further. Consistent with the outside results, but in contrast to our FISH results, the initial and repeat qRT-PCR results for both the peripheral blood and bone marrow samples were negative, despite robust amplification of a control gene (ABL).
Figure 1.
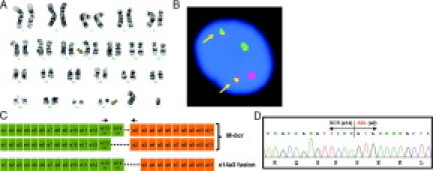
Summary of cytogenetic and molecular studies. A: G-banded karyotype. The t(9;22) is indicated by arrows. B: FISH analysis using LSI BCR/ABL dual fusion FISH probes BCR (22q11, SpectrumGreen) and ABL (9q34, SpectrumOrange). The1O1G2F pattern is typical of a reciprocal t(9;22). The two fusions are indicated with arrows. C: Schematic of the common M-bcr BCR-ABL fusion transcripts (e13a2 and e14a2) and the rare e14a3 transcript. The arrows indicate the position of the primers in the Ipsogen and Cepheid assays. D: DNA sequencing of the e14a3 fusion transcript identified in our patient.
Our clinical laboratory currently uses a commercially available assay (Mbcr/ABL FusionQuan kit, Ipsogen) for BCR-ABL qRT-PCR. This assay uses primers in exon 13 (e13) of the BCR gene and exon 2 (a2) of the ABL gene to detect e13a2 and e14a2 fusion transcripts (Figure 1C). To identify the BCR-ABL breakpoint in this patient, we performed RT-PCR using additional primer sets including forward primers in BCR exon1 (e1), BCR exon12 (e12), and a reverse primer in ABL exon3 (a3). These primer sets cover most of the previously reported uncommon breakpoints.7 The touchdown PCR reaction successfully amplified a 272-bp PCR product with the e12 and a3 primer pair, which suggested an e14a3 fusion (data not shown). Cycle sequencing of the 272 bp PCR confirmed the e14a3 fusion mRNA sequence, indicating that the ABL breakpoint was in intron 2 (Figure 1D). Although our routine qRT-PCR assay was unable to detect this rare fusion, FISH was able to detect this translocation and would detect other uncommon breakpoints because of the large size of the probes used. The LSI BCR/ABL dual color, dual fusion translocation probe set (Abbott Molecular Inc) covers the entire ABL and upstream ASS genes, spanning a genomic target of 650 kb, and the entire BCR gene as well as its upstream and downstream genomic regions spanning 1.5 Mb. Hence, these probes should be able to detect all BCR-ABL rearrangements, including cases with deletions around the breakpoints on the derivative 9, which can result in an apparently normal karyotype.8
BCR-ABL fusion transcripts with intronic breakpoints downstream of ABL a2, therefore lacking ABL exon 2, are rare, with a theoretically predicted incidence of 0.3% assuming that the breakpoints in ABL are equally distributed.9 So far only five cases of CML with e1a3, 10 cases of e13a3, and five cases of e14a3 BCR-ABL transcripts have been reported.10,11 These rare transcripts are also found in acute lymphocytic leukemia, with six cases reported previously.11 These uncommon transcripts may be under-reported, since RT-PCR-based assays may fail to detect these fusions due to the location of the primers and probes used.12,13 In our clinical experience, we have processed specimens from approximately 450 individuals with CML in the last 4 years. This is the first case in which we have failed to identify BCR-ABL transcripts with the Mbcr/ABL FusionQuant qRT-PCR assay in a specimen that was >90% positive for BCR-ABL fusion by FISH.
Biologically, BCR-ABL a3 type transcripts lack part of the ABL SH3 domain, which is present in the typical BCR-ABL a2 fusion transcripts. The ABL SH3 domain is believed to contribute to leukemogenesis by negatively regulating the kinase domain (SH1) and activating STAT5 signaling.14,15 Thus it is possible that CML with BCR-ABL a3 fusion transcripts might have a different clinical manifestation compared with CML with the common BCR-ABL a2 fusion transcripts. The BCR-ABL a3 breakpoint does not alter the sequence coding for the ATP/imatinib binding domain, but alterations in tertiary structure compared with a typical a2 fusion could affect drug response. The clinical outcomes specific to CML patients with BCR-ABL a3 fusions are difficult to define because of the limited number of cases reported. Three reported cases appeared to respond well to treatment with Gleevec (imatinib) and had a rather typical clinical progression.11 Our patient also appeared to have a good response to, although poor tolerance of, treatment with Gleevec. He was treated with Gleevec for a total of 30 days (20 days at 400 mg/day followed by 10 days at 300 mg/day). A follow-up BCR-ABL FISH analysis demonstrated a markedly reduced BCR-ABL fusion rate of 33.5%, indicating a partial cytogenetic response.16 Molecular response could not be assessed due to the persistently negative qRT-PCR results. The patient later went on Sprycel (dasatinib) at 20 mg a day for 2 months and, due to poor tolerance, was eventually changed to Hydrea (hydroxyurea). Certainly more reports on this rare transcript are needed to establish its clinical importance, if any.
While FISH and cytogenetics are good methods for identifying the BCR-ABL translocation in patients with CML, qRT-PCR is the standard test for minimum residual disease monitoring of CML patients receiving tyrosine kinase inhibitor therapy. Used in combination, the discrepancy between the results of the two methods was easily identified. However, if only the qRT-PCR assay had been used to monitor disease response/progression after treatment, and not used in conjunction with FISH or cytogenetics at the time of diagnosis, the negative result would have been misinterpreted as molecular remission. To address this problem, some clinical laboratories have developed their own multiplex RT-PCR assays for clinical detection of rare BCR-ABL transcripts.7,13
According to the March 2008 College of American Pathology BCR-ABL minimal residual disease (MRD) proficiency test report, approximately 45% of the 82 participating clinical laboratories reported using commercially designed primers and probes for BCR-ABL qRT-PCR. Of these laboratories, the reagents available from Roche (Indianapolis, IN) were the most common (approximately 39%), followed by reagents from Ipsogen (New Haven, CT) (25%), and Cepheid (Sunnyvale, CA) and Applied Biosystems (Foster City, CA) (14% each).17 Each of these assays uses a slightly different technical approach to BCR-ABL detection. Both the Cepheid and Ipsogen assays use primer sets located in exon 13 of BCR and exon 2 of ABL, which allow for the detection of the common e13a2 and e14a2 translocations (M-bcr, p210) but fail to amplify the rare variants lacking ABL exon 2 (BCR-ABL a3 type transcripts) (Figure 1C). The Roche assay also uses a forward primer in exon 13 of BCR, but uses a reverse primer in exon 4 of ABL, allowing for detection of these rare ABL a3 breakpoints. Applied Biosystems offers several different BCR-ABL assays, some of which would detect ABL a3 transcripts.
In summary, CML with a BCR-ABL a3 fusion is a rare and challenging entity. This breakpoint can lead to negative qRT-PCR results, which could be erroneously interpreted. Adhering to the current National Comprehensive Cancer Network practice guidelines (v2.2009), which recommends cytogenetics, FISH, and qRT-PCR as part of the initial workup for chronic phase adult CML, should avoid this problem. Although somewhat redundant, each assay can provide unique information. As mentioned, FISH and standard cytogenetics will identify uncommon BCR-ABL translocations that may be missed by qRT-PCR. Karyotyping will identify other cytogenetic abnormalities that may have prognostic significance. qRT-PCR allows for the specific breakpoint to be identified and also provides a baseline for reporting log reduction values for subsequent specimens. Once a complete cytogenetic response has been obtained, the National Comprehensive Cancer Network guidelines recommend qRT-PCR every 3 to 6 months, which unfortunately for patients with rare breakpoints may not be useful. Additional studies are needed to clarify natural incidence and unique clinical manifestations of this uncommon BCR-ABL fusion in patients with CML.
Acknowledgements
We thank the Kennedy Krieger Institute Cytogenetics Laboratory, the Johns Hopkins University Cancer Cytogenetics Laboratory, and the Johns Hopkins University Molecular Diagnostics Laboratory.
References
- 1.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 2.Chissoe SL, Bodenteich A, Wang YF, Wang YP, Burian D, Clifton SW, Crabtree J, Freeman A, Iyer K, Jian L. Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics. 1995;27:67–82. doi: 10.1006/geno.1995.1008. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, Cortes J, Albitar M, Arlinghaus R, Qiang GJ, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia with p185 (BCR/ABL) expression: characteristics and clinical significance. Br J Haematol. 1999;107:581–586. doi: 10.1046/j.1365-2141.1999.01736.x. [DOI] [PubMed] [Google Scholar]
- 4.Pane F, Frigeri F, Sindona M, Luciano L, Ferrara F, Cimino R, Meloni G, Saglio G, Salvatore F, Rotoli B. Neutrophilic-chronic myeloid leukemia: A distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction) Blood. 1996;88:2410–2414. [PubMed] [Google Scholar]
- 5.Barnes DJ, Melo JV. Cytogenetic and molecular genetic aspects of chronic myeloid leukemia. Acta Haematologica. 2002;108:180–202. doi: 10.1159/000065655. [DOI] [PubMed] [Google Scholar]
- 6.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chasseriau J, Rivet J, Bilan F, Chomel JC, Guilhot F, Bourmeyster N, Kitzis A. Characterization of the different BCR-ABL transcripts with a single multiplex RT-PCR. J Mol Diagn. 2004;6:343–347. doi: 10.1016/S1525-1578(10)60530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista DA, Hawkins A, Murphy KM, Griffin CA. BCR/ABL rearrangement in two cases of Philadelphia chromosome negative chronic myeloid leukemia: deletion on the derivative chromosome 9 may or not be present. Cancer Genet Cytogenet. 2005;163:164–167. doi: 10.1016/j.cancergencyto.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 9.van der Plas DC, Soekarman D, van Gent AM, Grosveld G, Hagemeijer A. Bcr-Abl mRNA lacking abl exon a2 detected by polymerase chain reaction in a chronic myelogeneous leukemia patient. Leukemia. 1991;5:457–461. [PubMed] [Google Scholar]
- 10.Liu L-G, Tanaka H, Ito K, Kyo T, Ito T, Kimura A. Chronic myelogenous leukemia with e13a3 (b2a3) type of BCR-ABL transcript having a DNA breakpoint between ABL exons a2 and a3. Am J Hematol. 2003;74:268–272. doi: 10.1002/ajh.10429. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa S, Nakamura S, Naito K, Kobayashi M, Ohnishi K. A variant transcript, e1a3, of the minor BCR-ABL fusion gene in acute lymphoblastic leukemia: case report and review of literature. Int J Hematol. 2008;87:184–188. doi: 10.1007/s12185-008-0031-5. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ali HK, Leiblein S, Kovacs I, Hennig E, Niederwieser D, Deininger MW. CML with an e1a3 BCR-ABL fusion: rare, benign, and a potential diagnostic pitfall. Blood. 2002;100:1092–1093. doi: 10.1182/blood-2002-03-0930. [DOI] [PubMed] [Google Scholar]
- 13.Wilson GA, Vandenberghe EA, Pollitt RC, Rees DC, Goodeve AC, Peake IR, Reilly JT. Are aberrant BCR-ABL transcripts more common than previously thought? Br J Haematol. 2000;111:1109–1111. doi: 10.1046/j.1365-2141.2000.02471.x. [DOI] [PubMed] [Google Scholar]
- 14.Skorski T, Nieborowska-Skorska M, Wlodarski P, Wasik M, Trotta R, Kanakaraj P, Salomoni P, Antonyak M, Martinez R, Majewski M, Wong A, Perussia B, Calabretta B. The SH3 domain contributes to BCR/ABL-dependent leukemogenesis in vivo: role in adhesion, invasion, and homing. Blood. 1998;91:406–418. [PubMed] [Google Scholar]
- 15.Nieborowska-Skorska M, Wasik MA, Slupianek A, Salomoni P, Kitamura T, Calabretta B, Skorski T. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J Exp Med. 1999;189:1229–1242. doi: 10.1084/jem.189.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 17.Minimal Residual Disease MRD-A Participant Summary. College of American Pathologists; 2008. p. 17. [Google Scholar]


