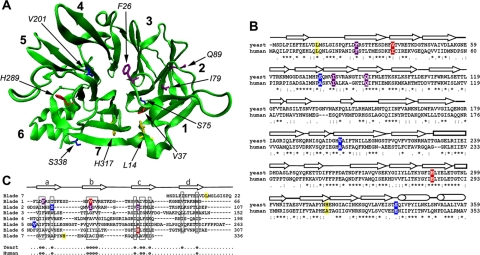
Figure 1.
Structure of the clathrin heavy chain TD. (A) A homology model of the yeast TD (green) with blades 1–7 of the β-propeller numbered in bold. Side chains from amino acid residues that were mutated in the chc1-TD-ts alleles and the chc1-box allele are shown and color coded for each allele as follows: chc1-1 and chc1-3 (dark blue), chc1-2 (red), chc1-4 (yellow), and chc1-box (purple). (B) Sequence alignment of the yeast TD (residues 1-359) and human TD (residues 1-353) regions of clathrin heavy chain. Residues that were mutated in the chc1-TD-ts alleles and the chc1-box allele are highlighted according to the same color scheme used in A. An asterisk (*) denotes identical residues between the yeast and human sequence, a colon (:) denotes a conserved substitution between sequences, and a period (.) denotes a semiconserved substitution between sequences. (C) Alignment of the seven blades of the TD β-propeller structure. The approximate boundaries for every strand in each blade are denoted above the alignment. θ denotes conserved hydrophobic residues in both the yeast and human sequence. The S75P mutation of chc1-1 is also present in chc1-3.