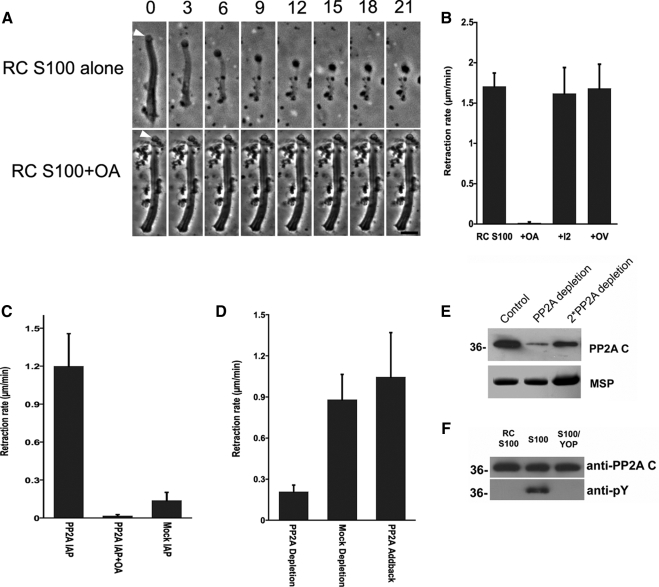
Figure 3.
Intrinsic PP2A triggers retraction and is regulated by tyrosine dephosphorylation. (A) Time-lapse sequences showing retraction induced by perfusion of fibers with RC-S100 (top). OA blocked this retraction (bottom). Bar, 5 μm. (B) Comparison of rates of retraction induced by RC-S100 alone or with added OA (10 nM), OV (1 mM), or I2 (100 ng/μl). Entries represent the mean retraction rate for 10–15 fibers ± SD. (C) Rate of retraction induced by sperm PP2A, isolated by immunoprecipitation, in the presence and absence of OA. Material obtained by mock IP with mouse IgG served as a control. Entries are the means of seven to 10 fibers ± SD. (D) Effect of PP2A depletion on the rate of retraction induced by RC-S100. PP2A-depletion reduced the rate of retraction to ∼23% of that obtained using mock-depleted extracts. Add-back of a commercially obtained PP2A restored the retraction-triggering activity of depleted extracts. (E) SDS-PAGE and Western blot analysis of immunodepleted S100. Top, portion of a Western blot probed with anti-PP2Ac. Bottom, portion of an equivalent Coomassie Blue-stained gel containing MSP to show the relative amounts of sample loaded in each lane. The untreated control (left) and PP2A-depleted (center) lanes were loaded with equal amounts of total protein; the right lane was loaded with twofold more sample. Approximately 80% of PP2A was removed by immunodepletion. (F) SDS-PAGE gels of PP2A immunoprecipitated from RC-S100 (left), S100 (center), and YOP-treated S100 (right) and immunoblotted with either anti-PP2Ac (top) or anti-pY antibody (bottom). The PP2A from S100 was labeled by anti-pY but that from RC-S100 was not. YOP treatment abolished anti-pY labeling of the PP2A from S100.