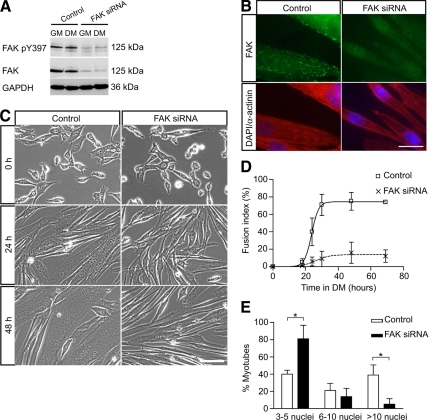
Figure 4.
Down-regulation of FAK protein expression by siRNA inhibits myoblast fusion. (A) Myoblasts were transfected with control siRNA or FAK siRNA, cultured in DM for 48 h and then analyzed by Western blots to determine the efficacy of FAK knockdown by siRNA. The levels of phosphorylated FAK and FAK protein were dramatically down-regulated in FAK siRNA-treated cells. GAPDH was used as a loading control. (B) Immunofluorescence of siRNA-treated myotubes with an anti-FAK antibody revealed the presence of FAK at focal adhesions in control cells but not in FAK siRNA-treated cells. Coimmunostaining for sarcomeric α-actinin showed expression of this marker of muscle terminal differentiation in FAK-inhibited cells. Nuclei are labeled with DAPI. Bar, 20 μm. (C) Myoblasts transfected with control or FAK siRNA were cultured in DM to determine the effect of FAK down-regulation on myoblast fusion. Myoblast fusion was impaired in FAK siRNA-treated cultures in which most cells were elongated but remained mono- or binucleated. Bar, 100 μm. (D) Determination of the fusion index in control and FAK siRNA-treated cultures confirmed the defect of myoblast fusion when FAK was inhibited. (E) Determination of myonuclear content in control and FAK siRNA-treated cultures. Cells transfected with siRNA oligonucleotides were cultured in DM for 48 h. The proportion of myotubes containing few nuclei was increased whereas the proportion of myotubes containing a large number of nuclei was decreased in FAK siRNA-treated cultures (*p < 0.05).