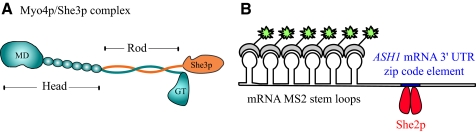
Figure 1.
(A) Schematic of the proposed complex between Myo4p (cyan) and She3p (orange). The Myo4p head consists of the motor domain (MD) and six IQ motifs to which calmodulin is bound. The rod region is proposed to be a heterocoiled-coil between Myo4p and the adapter protein She3p (Hodges et al., 2008). The globular tail of Myo4p follows the rod. (B) Diagram of the reporter system used to observe fluorescently labeled ASH1 mRNA in living yeast (Bertrand et al., 1998). The 3′ UTR region of ASH1 is joined to six MS2 stem loop binding motifs for the MS2 coat protein. Each stem loop binds one MS2-GFP molecule. Unbound MS2-GFP is confined to the nucleus. The region of ASH1 mRNA used in this reporter contains one zip code element, which binds one homodimeric molecule of She2p (red), based on the work of Niessing et al. (2004).