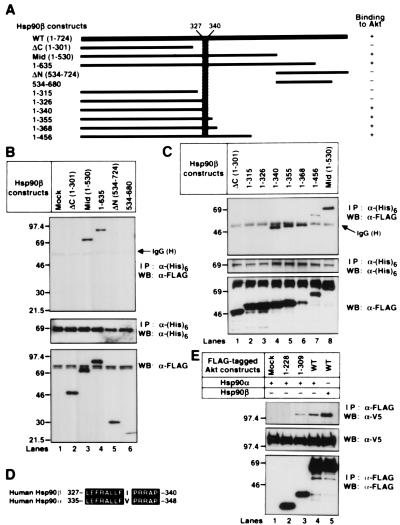
Figure 3.
Identification of the Hsp90β domain responsible for binding to Akt. (A) Structural domains of Hsp90β and Hsp90β deletion mutants used in these experiments are represented as black bars. The predicted Akt binding site in Hsp90β is shown schematically (hatched). (B and C) The (His)6-tagged WT-murine akt was transiently transfected into 293T cells together with mock or the indicated FLAG-tagged hsp90β mutants. The (His)6-tagged proteins were immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with an anti–FLAG M2 mAb. Molecular size markers are indicated (in kDa). (D) Alignment of the amino acid sequence of the human Hsp90β with the equivalent region of human Hsp90α. Identical residues are denoted by white letters on black background. The GenBank accession nos. of human Hsp90β and human Hsp90α were M16660 and X15183, respectively. (E) The V5-tagged WT-hsp90α or WT-hsp90β was transiently transfected into 293T cells together with mock or the indicated FLAG-tagged akt mutants. The FLAG-tagged proteins were immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with an anti-V5 mAb. Molecular size markers are indicated (in kDa).