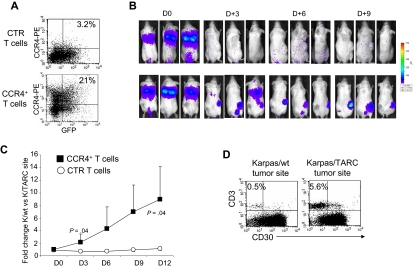
Figure 5.
Improved in vivo migration of CCR4+ T cells. CTR and CCR4+ T cells were transduced to express eGFP-FFLuc to monitor their migration in vivo in SCID mice, using the IVIS imager system. (A) The expression of eGFP-FFLuc on CTR (top plot) and CCR4+ (bottom plot) T cells evaluated by GFP. (B) The bioluminescence signal from CTR and CCR4+ T cells in 3 representative SCID mice/group engrafted with TARC− tumor (K/wt) on the left side and the TARC+ tumor (K/TARC) on the right side. Whereas no significant expansion of the bioluminescent signal was observed to either site of tumor in mice receiving CTR T cells (top picture in each pair), an increase in bioluminescence was observed in mice receiving CCR4+ T cells (bottom picture in each pair) only at the site of the tumor producing TARC. (C) The fold change of bioluminescence signal between K/wt and K/TARC sites for CTR (○) and CCR4+ (■) T cells. Data are mean ± SD of 6 mice. (D) The immunophenotype of K/wt (left plot) and K/TARC (right plot) tumors isolated from one representative mouse that received CCR4+ T cells, killed on day 9. After removal, the tumors were homogenized and cells stained to distinguish T cells (using an antihuman CD3 antibody) from Karpas tumor cells (using an antihuman CD30 antibody). As shown in the panel, the proportion of cells detectable at the site of tumor-secreting TARC (5.6%) was more than 10-fold higher compared with K/wt (0.5%). This correlated with the increase in bioluminescence signal at the site of K/TARC tumor (2.1 × 106 p/sec/cm2/sr) compared with the site of K/wt tumor (1.4 × 105 p/sec/cm2/sr).