Fig. 4.
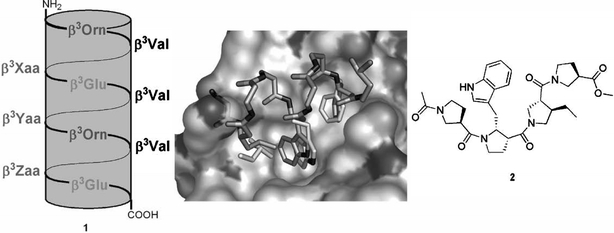
Macrodipole-stabilised 14-helical β3-peptides 1 as p53–HDM2 antagonists. Different combinations of the F19, W23, and L26 side chains were introduced at the positions β3-Xaa, β3-Yaa, and β3-Zaa. The best solution was Leu, Trp, and Phe at Xaa, Yaa, and Zaa, respectively (see text). A model of this peptide, assuming an ideal 14-helical structure, was build (dark sticks; only interacting side chains shown) and overlaid with the known interaction of a p53 peptide (only key side chains shown as light sticks) with HDM2 (surface). The residue preceding ß3-Leu clashes with the H96 imidazole, although it can easily be imagined that the H96 side chain moves to allow access. Constructed from PDB # 1YCR. A hypothetical peptidomimetic of the p53 peptide residue triad based on a β-Pro oligomer (2) has also been proposed.
