Figure 1. Expression and anti-Bax function of full-length PrPΔBOR4 and PrPΔBOR4/BH2 mutants.
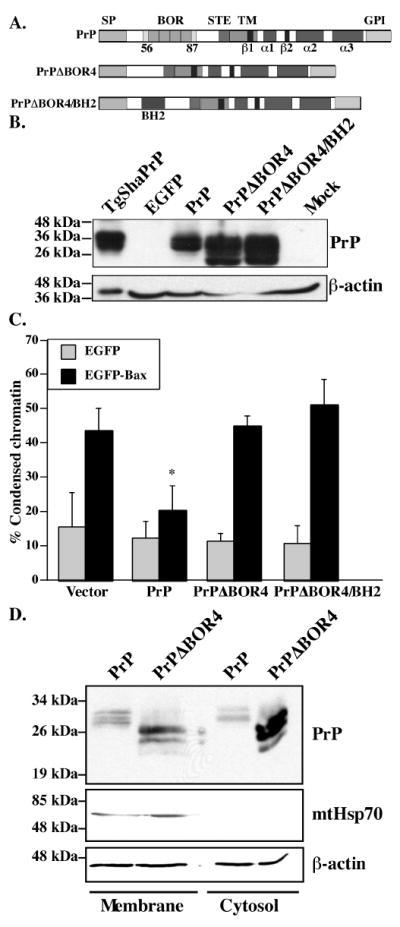
A. Schematic diagram showing the main domains and structural elements of PrP, PrPΔBOR4 and PrPΔBOR4/BH2. Abbreviations: SP: N-terminal ER-targeting signal peptide (residues 1-23), BOR: BH2-like octapeptide repeats (residues 56-87), as opposed to standard octapeptide repeats (residues 51-91), STE: Stop-transfer effector sequence (residues 104-111), TM: transmembrane domain (residues 112-135), β1 and β2: beta-strands 1 (residues 128-131) and 2 (residues 161-164), α1, α2 and α3: alpha-helices 1 (residues 144-154), 2 (residues 173-194) and 3 (residues 200-227), GPI: glycosyl phosphatidylinositol anchor signal (residues 232-253). B. Western blot of 100 μg SDS-soluble proteins extracted from EGFP, PrP, PrPΔBOR4, PrPΔBOR4/BH2, or Mock-transfected cells with 3F4 anti-PrP and anti-β-actin antibodies. Proteins (1.05 μg) extracted from Syrian Hamster PrP transgenic mice brains (TgSHaPrP) were used as a positive control for PrP immunodetection. C. Percentage of cell death assessed by chromatin condensation in MCF-7 cells transfected with pBud-EGFP, pBud-EGFP/PrP, /PrPΔBOR4, or /PrPΔBOR4/BH2, and pBud-EGFP-Bax, pBud-EGFP-Bax/PrP, /PrPΔBOR4 or /PrPΔBOR4/BH2. Data represents the mean ± standard error of the mean (SEM) of 3 independent experiments. At least 100 cells were counted in each experiment. Asterisk sign indicates a statistically significant difference from pBud-EGFP or pBud-EGFP-Bax (p≤0.05). D. Western blot analysis of proteins from membrane and cytosolic subcellular fractions with 3F4, mtHsp70 and β-actin antibodies.
