Figure 13.
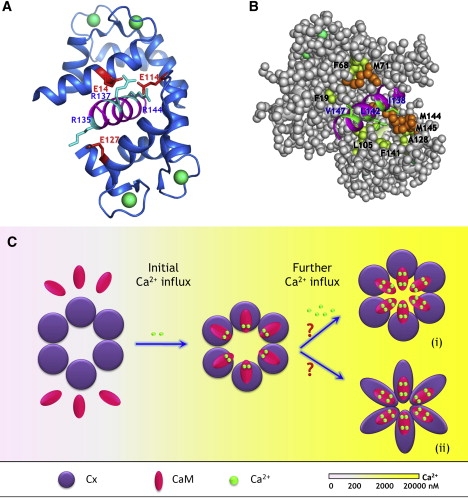
Proposed model of Ca2+-mediated regulation of gap junction permeability. (A and B) Modeled structure of the CaM-Cx44129–150 complex. The model structure is built based on the 3D structure of holo-CaM in complex with the CaM binding region from CaMKII (pdb entry: 1cdm) using MODELER. Residues involved in potential (A) electrostatic interactions and (B) hydrophobic interactions were indicated. Specifically, residues R135, R137, and R144 from the peptide (cyan) are capable of forming salt bridges with residues E127, E14, and E114 (red) from holo-CaM, respectively. In addition, hydrophobic residues from the peptide (V147, V142, and I138, green) is in close proximity with F19/F68/M71, and L105/F141, and A128/M144/M145 from holo-CaM (Met, orange; Phe and Ala, yellow), respectively. (C) Proposed model of regulation of gap junction inhibition mediated by CaM. The subtle changes in the intracellular Ca2+ concentration (at submicromolar range) are initially sensed by the “high-affinity” C-domain of CaM, enabling it to preferably interact with the intracellular loop of Cx proteins. Such interaction enhances the efficiency and sensitivity of intracellular Ca2+ sensing because of increases in both Ca2+-binding affinity and intradomain cooperativity within the C-domain of CaM. The partially saturated, Cx-bound CaM might serve as an intermediate state to prevent the free diffusion of CaM in the cytoplasm. Once the intracellular Ca2+ is further elevated to micromolar range, the half-saturated CaM is then able to quickly respond to the Ca2+ signals and triggers the fully open conformation that is capable of leading to the inhibition of intercellular communication mediated by gap junction. The entity responsible for the Ca2+-mediated inhibition of gap junction still remains to be defined. It could arise from the physical obstruction of the pore by holo-CaM (state i), or be due to the holo-CaM triggered conformational changes in Cx protein itself (state ii). Whether or not all six subunits have to bind calmodulin to exhibit Ca2+-dependent gap junction inhibition remains to be defined.
