Figure 5.
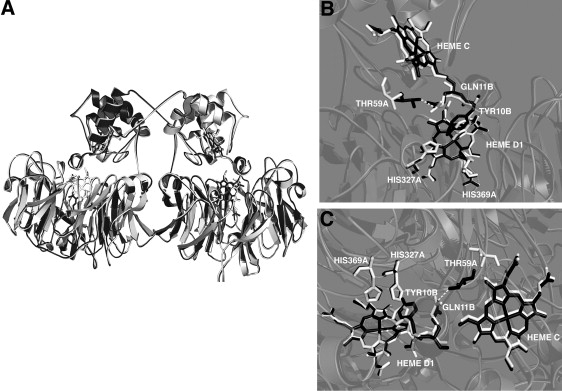
Conformational changes occurring upon reduction of P. aeruginosa-NiR. (A) Superposition of the 3D structure of the oxidized (white) and reduced (black) dimeric enzyme, highligthing the swapping of the N-termini. Two different views (B and C) of the interface between the heme-c and heme-d1 domains in the two oxidation states are presented. Three relevant residues in the heme-d1 pocket are depicted: His-369 and His-327 belonging to monomer A (HIS-327A and HIS-369A), and Tyr-10 coming from the other monomer B (TYR-10B). The reorganization of the 56-62 loop and the new H-bond (dashed line) between Thr-59 (from monomer A) and Gln-11 (from monomer B) formed upon reduction are shown (THR-59A and GLN-11B).
