Figure 5.
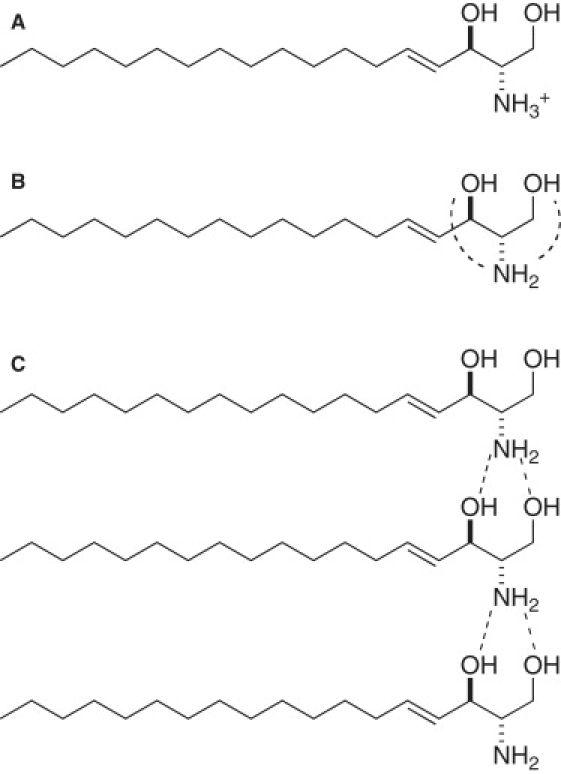
Predominant molecular states of aggregated sphingosine at different pH values suggested by the data shown in this study. (A) At pH below pKa (6.6), NH2 moiety of sphingosine is protonated. At pH slightly above pKa, sphingosine exists as an electrically neutral molecule with a deprotonated amine group. (B) As reported by Merrill et al. (31), intramolecular hydrogen bonds are formed between OH and NH2 groups of sphingosine (broken lines). (C) Between pH 6.7 and 9.9, intermolecular hydrogen bonding becomes predominant (broken lines); such bonds are efficiently formed only when sphingosine molecules exist in close proximity, which increases the mean aggregate radius.
