Figure 3.
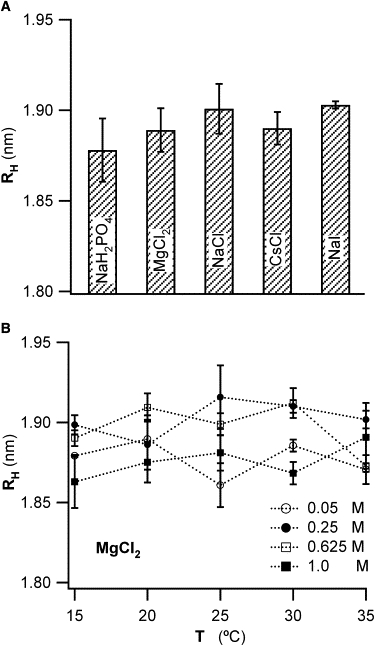
Effects of chaotropic and kosmotropic salt ions on lysozyme hydration. (A) Mean hydrodynamic radius RH of lysozyme in the presence of various salts with predominately chaotropic or kosmotropic salt ions. RH values were derived from the measured free particle diffusivity D0 (see Fig. 1) and corrected for the salt- and temperature-dependent changes in water viscosity (see Fig. 2 and Table 1). RH values for different concentrations of the same salt were averaged because they displayed no discernible systematic variations (B). For comparison, the thickness of a monolayer of water is ∼0.26–0.28 nm. (B) Hydrodynamic radius RH of lysozyme in the presence of MgCl2 at different solution temperatures T, and for MgCl2 concentrations ranging from 50 mM to 1 M. The lack of any systematic variation with temperature or salt concentration is representative for our measurements with any salts, and at all salt concentrations and solution temperatures.
