Figure 1.
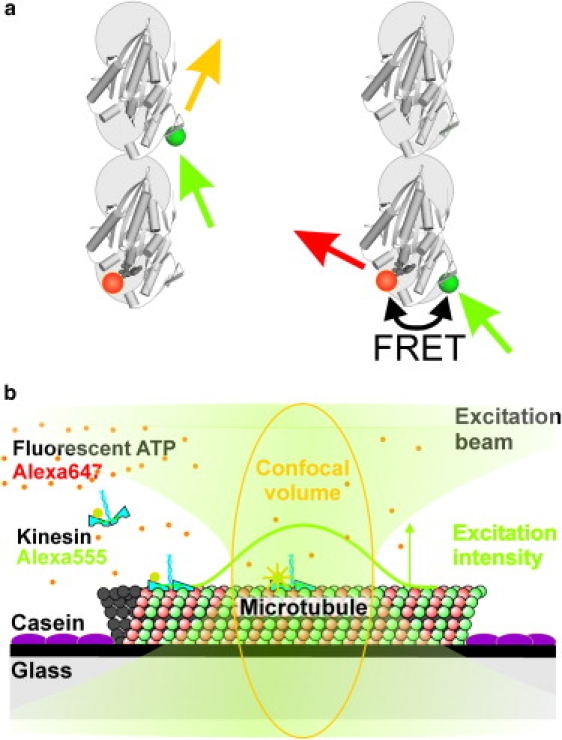
Schematic representation of the experimental setup. (a) Molecular model of FRET between S43C-labeled kinesin and fluorescent nucleotide. Two microtubule-bound motor domains (PDB: 2KIN (27)), one of which is labeled by Alexa Fluor 555 (green dots), are depicted from the top (+ end upward). The acceptor-labeled nucleotide (orange dots) is bound to the rearward head. Negligible FRET efficiencies are expected in the situation shown on the left, where fluorescent nucleotide is bound to the leading head. The close proximity of S43C to the nucleotide-binding pocket gives rise to efficient FRET when Alexa Fluor 555 label and fluorescent ATP are at the same motor domain (right). (b) Schematic representation of the single-motor FRET assay based on confocal fluorescence microscopy. A microtubule is attached to a glass surface using charge interactions. The surface is blocked for nonspecific interactions with casein. The confocal spot of a fluorescence microscope is positioned on the microtubule. Alexa Fluor 555-labeled kinesins walk through the excitation spot and either emit or transfer the excitation to Alexa Fluor 647-labeled ATP bound to the motor. The fluorescence of both Alexa Fluor 555 and 647 is collected and detected.
