Figure 1.
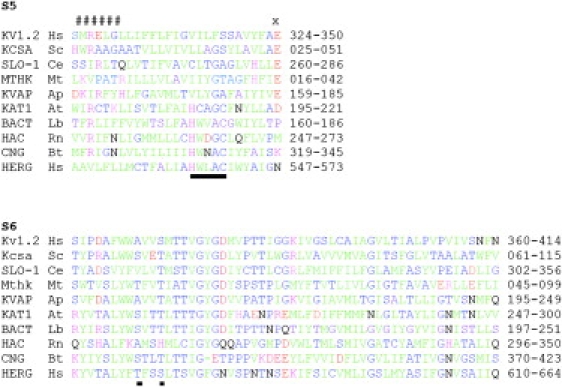
Amino acid sequence alignments of the S5 sequence alignment of HERG with KVAP. (A and B) For S5, CNBD channels were first aligned based on a conserved consensus HwX(A/G)C. This is underlined. All other channels were aligned using the conserved COOH-terminal glutamic acid (X). The two groups were aligned by trying to minimize the incursion of NH2-terminal basic residues (#####) into the membrane. The channels aligned are Homo sapiens Kv1.2 NP_004965, Streptomyces coelicolor Kcsa NP_631700, Caenorhabditis elegans SLOwpoke-1 NP_001024260, Methanothermobacter thermautotrophicus Mthk NP_276634, Aeropyrum pernix KVAP NP_147625, Arabidopsis thaliana KAT1 NP_199436, Leptospira biflexa bacterial CNBD channel ABZ94327, Rattus norvegicus hyperpolarization activated channel NP_446137, Bos taurus cyclic nucleotide gated channel NP_776704, and Homo sapiens HERG1 ABF71886. (C) Pore-S6 alignment for the above ion channels. Green, large nonpolar residues I, L, M, V, F, Y, and W. Blue, mainly small (except T) mainly polar (except A) residues A, C, G, S, and T. Black, large polar residues Q and N. Red, acidic residues D and E. Purple, basic residues K, R, and H.
