Figure 3.
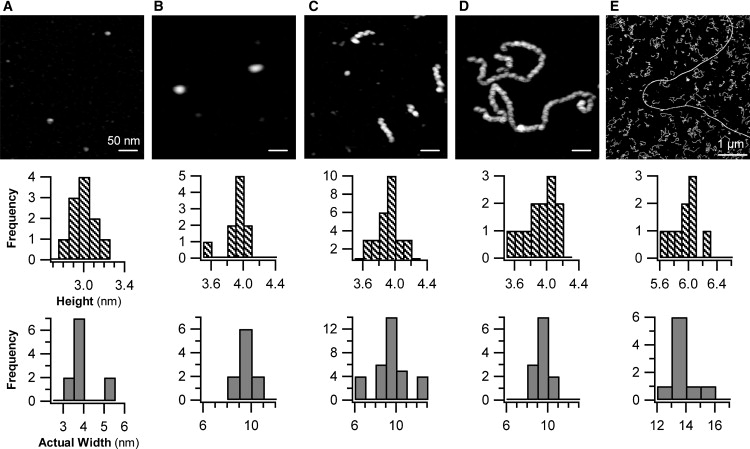
AFM images of intermediate amyloid aggregates formed by lysozyme. Evolution of aggregate morphologies for lysozyme amyloid growth at pH 2.0 and T = 50°C. (A) Before heating (T = 20°C), a uniform population of HEWL monomers is present. (B) AFM images of HEWL samples after 3 h of incubation at 50°C predominately yield oligomers. (C) AFM image of protofibrils appearing ∼10 h, i.e., shortly after nucleation event seen in DLS. (D) AFM image of protofibrils after 25 h of incubation. (E) AFM image of long, straight fibrils formed after ∼100 h of incubation, against a background of protofibrils (note change in scale-bar dimension). Below each image is the distribution of aggregate heights and lateral aggregate widths (the latter corrected for AFM tip distortions). Apparent difference in oligomer size in B versus protofibril width in C highlights effect of AFM scanning tips (16 nm in A and B versus 12.5 nm in C–E) on lateral aggregate dimensions. Tip-corrected aggregate dimensions are identical (see Fig. 5 and Table 1).
